
EXPEDITED PUBLICATION
Research Article
Movement Disorder Society-Sponsored Revision of the Unified
Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale
Presentation and Clinimetric Testing Results
Christopher G. Goetz,
1
*
Barbara C. Tilley,
2
Stephanie R. Shaftman,
2
Glenn T. Stebbins,
1
Stanley Fahn,
3
Pablo Martinez-Martin,
4
Werner Poewe,
5
Cristina Sampaio,
6
Matthew B. Stern,
7
Richard Dodel,
8
Bruno Dubois,
9
Robert Holloway,
10
Joseph Jankovic,
11
Jaime Kulisevsky,
12
Anthony E. Lang,
13
Andrew Lees,
14
Sue Leurgans,
1
Peter A. LeWitt,
15
David Nyenhuis,
16
C. Warren Olanow,
17,18
Olivier Rascol,
19
Anette Schrag,
20
Jeanne A. Teresi,
21
Jacobus J. van Hilten,
22
and Nancy LaPelle,
23
for the Movement Disorder Society UPDRS Revision Task Force
1
Department of Neurological Sciences, Rush University Medical Center, Chicago, Illinois
2
Biostatistics, Bioinformatics, and Epidemiology, Medical University of South Carolina, Charleston, South Carolina
3
Department of Neurology, Columbia University, New York, New York
4
Neuroepidemiology Unit and CIBERNED, Carlos III Institute of Health, Madrid, Spain
5
Department of Neurology, Innsbruck Medical University, Innsbruck, Austria
6
Department of Pharmacology, Faculdade de Medicina de Lisboa, Lisboa, Portugal
7
Department of Neurology, University of Pennsylvania, Philadelphia, Pennsylvania
8
Department of Neurology, Philipps-University, Marburg, Germany
9
Department of Neurology, Ho
ˆ
pital de la Salpe
ˆ
trie
`
re, Paris, France
10
Department of Neurology, University of Rochester, Rochester, New York
11
Department of Neurology, Baylor College of Medicine, Houston, Texas
12
Department of Neurology and CIBERNED, Sant Pau Hospital, Barcelona, Spain
13
Division of Neurology, University of Toronto, Toronto, Canada
14
Reta Lila Weston Institute of Neurological Studies, University College, London, England
15
Department of Neurology, Wayne State University, Detroit, Michigan
16
Department of Neurology and Rehabilitation, University of Illinois, Chicago, Illinois
17
Department of Neurology, Mount Sinai School of Medicine, New York, New York
18
Department of Neuroscience, Mount Sinai School of Medicine, New York, New York
19
Laboratoire de Pharmacologie Me
´
dicale et Clinique, Toulouse University, Toulouse, France
20
Department of Clinical Neurosciences, University College, London, England
21
Stroud Center, Division of General Medicine and Psychiatric Institute, Columbia University, New York, New York
22
Department of Neurology, Leiden University, Leiden, The Netherlands
23
Division of Preventive and Behavioral Medicine, University of Massachusetts, Worcester, Massachusetts
Potential conflict of interest: All authors have confirmed they have
no conflict of interest related to this effort.
Received 7 July 2008; Revised 23 August 2008; Accepted 3
September 2008
Published online 20 November 2008 in Wiley InterScience
(www.interscience.wiley.com). DOI: 10.1002/mds.22340
Additional Supporting Information may be found in the online
version of this article.
*Correspondence to: Dr. Christopher G. Goetz, Department of
Neurological Sciences, Rush University Medical Center, Suite 755,
1725 West Harrison Street, Chicago, IL 60612. E-mail: cgoetz@
rush.edu
2129
Movement Disorders
Vol. 23, No. 15, 2008, pp. 2129–2170
2008 Movement Disorder Society

Abstract: We present a clinimetric assessment of the
Movement Disorder Society (MDS)-sponsored revision of the
Unified Parkinson’s Disease Rating Scale (MDS-UPDRS).
The MDS-UDPRS Task Force revised and expanded the
UPDRS using recommendations from a published critique.
The MDS-UPDRS has four parts, namely, I: Non-motor
Experiences of Daily Living; II: Motor Experiences of Daily
Living; III: Motor Examination; IV: Motor Complications.
Twenty questions are completed by the patient/caregiver.
Item-specific instructions and an appendix of complementary
additional scales are provided. Movement disorder specialists
and study coordinators administered the UPDRS (55 items) and
MDS-UPDRS (65 items) to 877 English speaking (78% non-Lat-
ino Caucasian) patients with Parkinson’s disease from 39 sites.
We compared the two scales using correlative techniques and
factor analysis. The MDS-UPDRS showed high internal consis-
tency (Cronbach’s alpha 5 0.79–0.93 across parts) and corre-
lated with the original UPDRS (q 5 0.96). MDS-UPDRS across-
part correlations ranged from 0.22 to 0.66. Reliable factor struc-
tures for each part were obtained (comparative fit index > 0.90
foreachpart),whichsupporttheuseofsumscoresforeachpart
in preference to a total score of all parts. The combined clinimet-
ric results of this study support the validity of the MDS-UPDRS
for rating PD. 2008 Movement Disorder Society
Key words: Parkinson’s disease; rating scales; UPDRS;
clinimetrics
The Unified Parkinson’s Disease Rating Scale
(UPDRS) was originally developed in the 1980s
1
and
has become the most widel y used clinical rating scale
for Parkinson’s disease (PD).
2
In 2001, the Movement
Disorder Society (MDS) sponsored a critique of the
UPDRS, and this document lauded the strengths of the
scale but identified a numb er of ambiguities, weak-
nesses, and areas in need of inclusion to reflect current
scientific developments.
3
The summary conclusions
recommended the development of a new version of the
UPDRS that would retain the strengths of the original
scale, but resolve identified problems and especially
incorporate a number of clinically pertinent PD-related
problems poorly captured in the original version. Based
on this critique, the MDS commissioned a revision of
the scale, resulting in a new version, termed the MDS-
sponsored UPDRS revision (MDS-UPDRS).
4
This
scale successfully passed initial clinimetric testing
4
and
was therefore submitted to a large-scale comparison
with the original UPDRS. This report presents the
MDS-UPDRS for the first time in published form and
the clinimetric testing results of this large-scale pro-
gram among native English speaking PD patients.
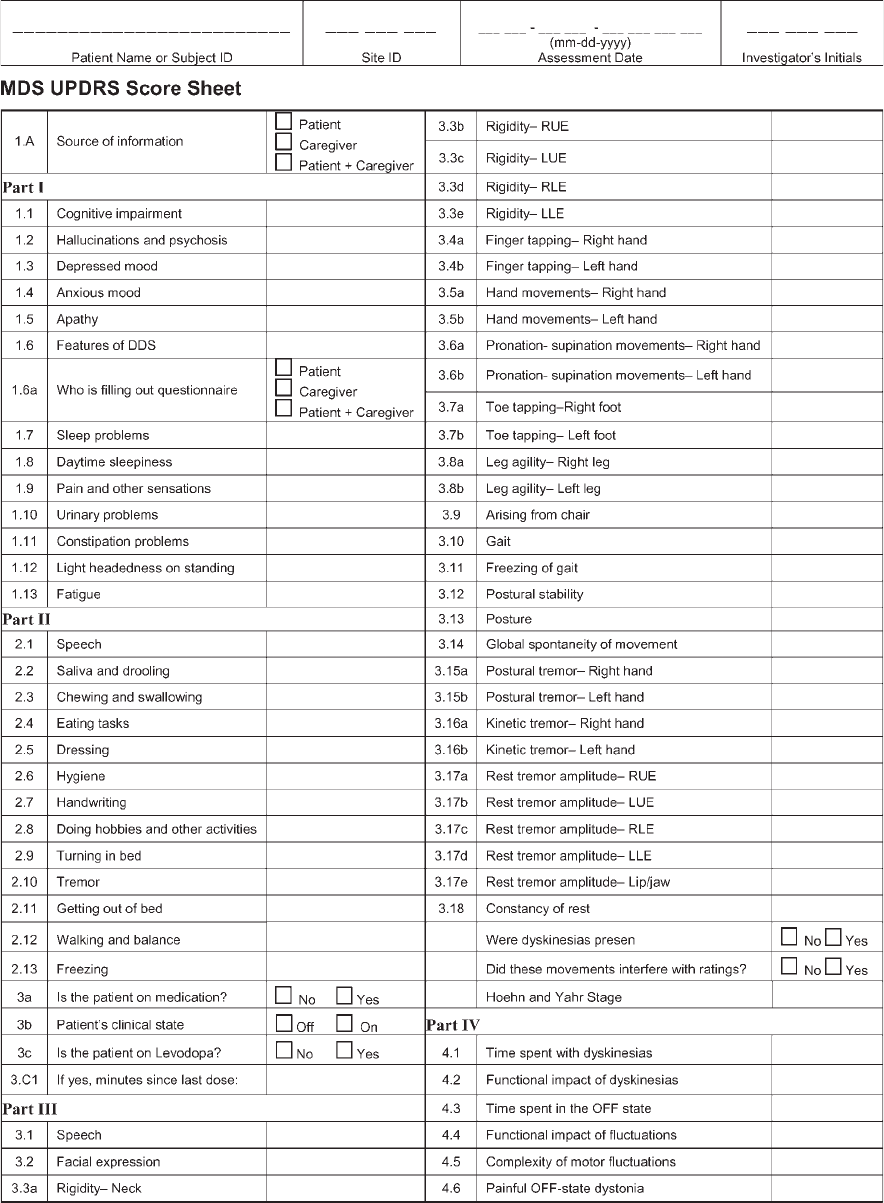
MDS-UPDRS (Table 1): The primary areas of revi-
sion were discussed in a prior publication.
4
First,
whereas the original four component (Parts I–IV)
design was retained, the focus of each part has been
changed, and the data acquisition methodology has
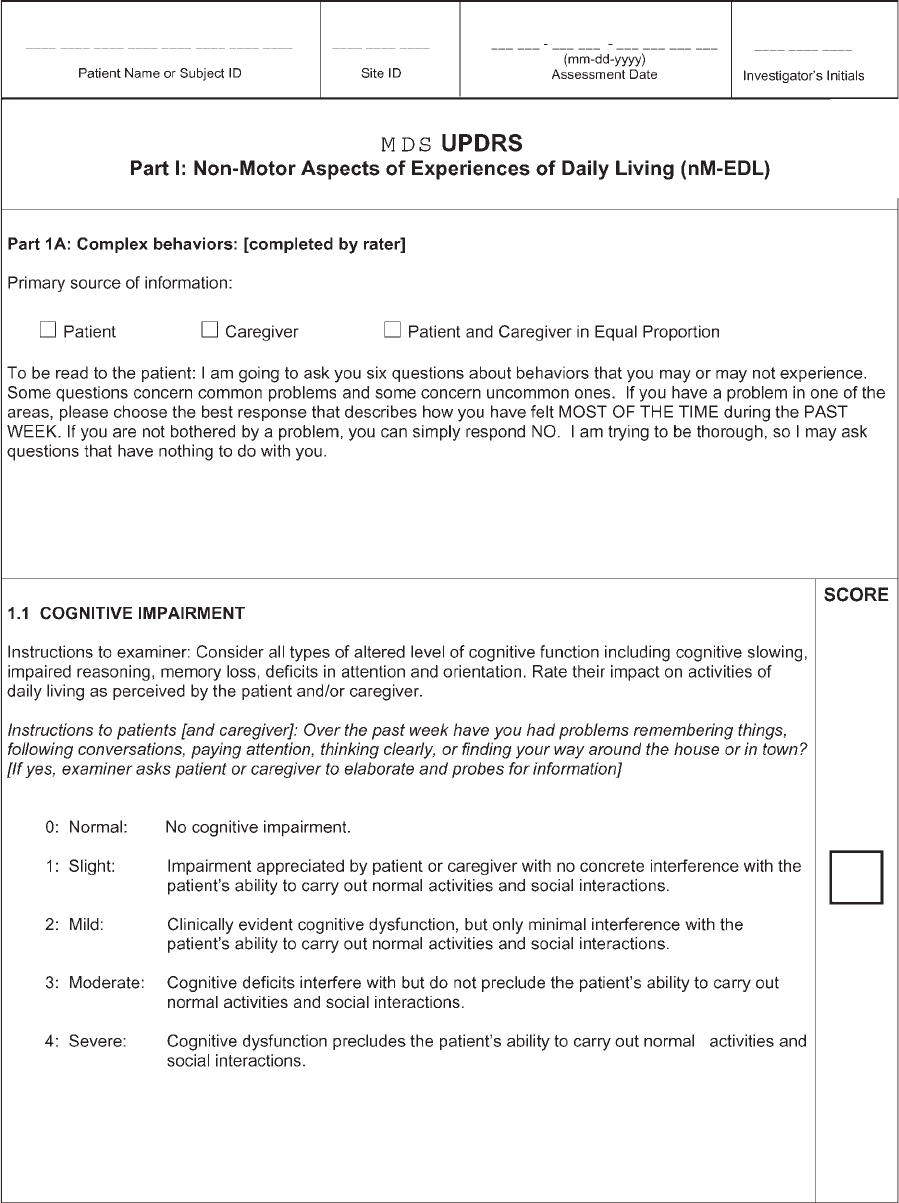
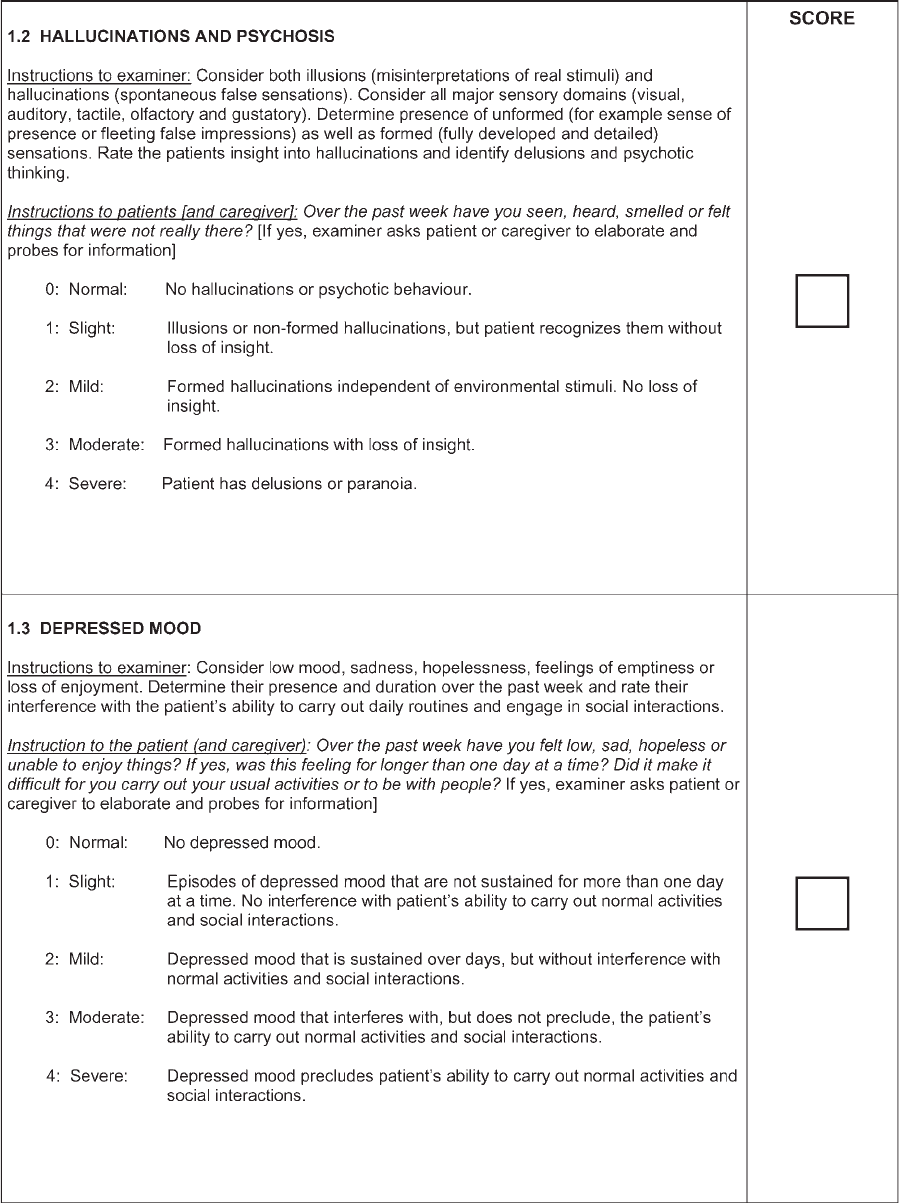
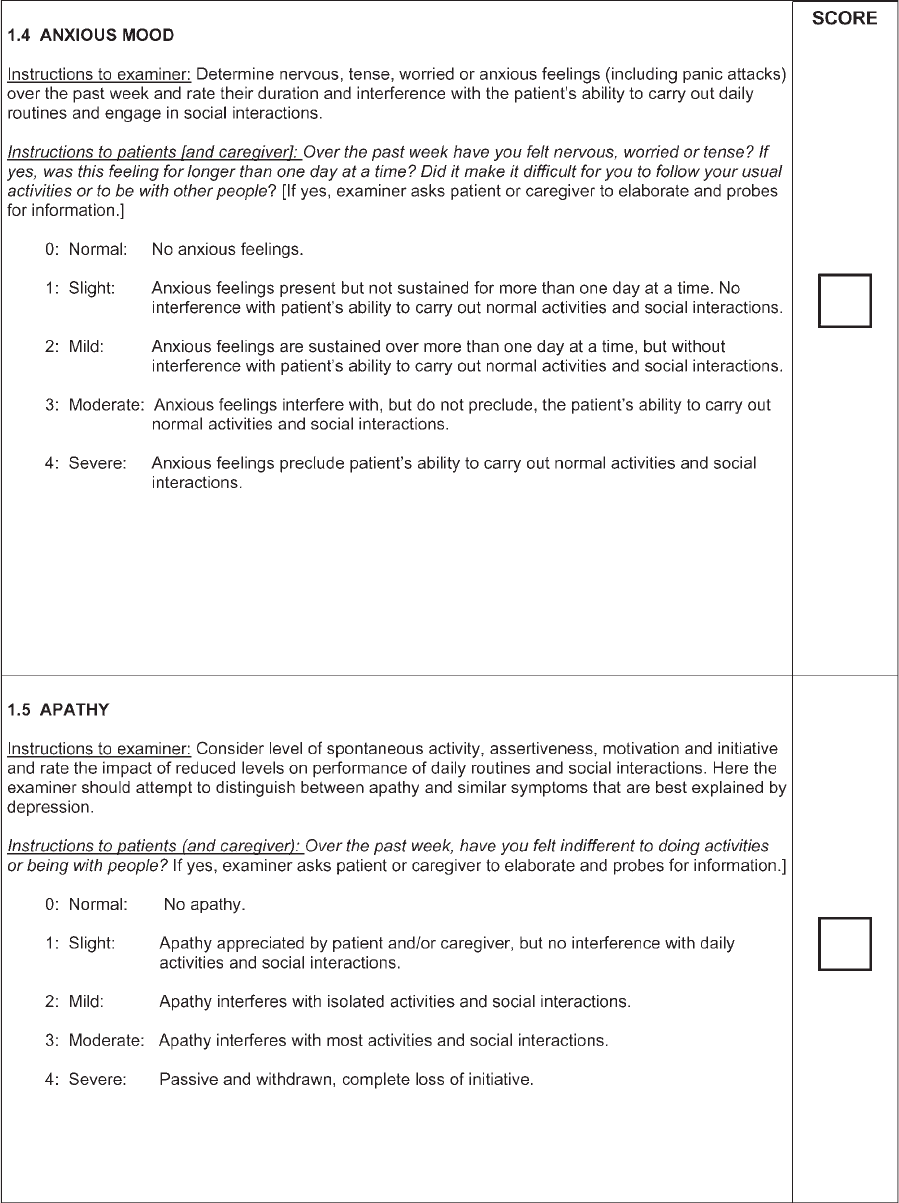
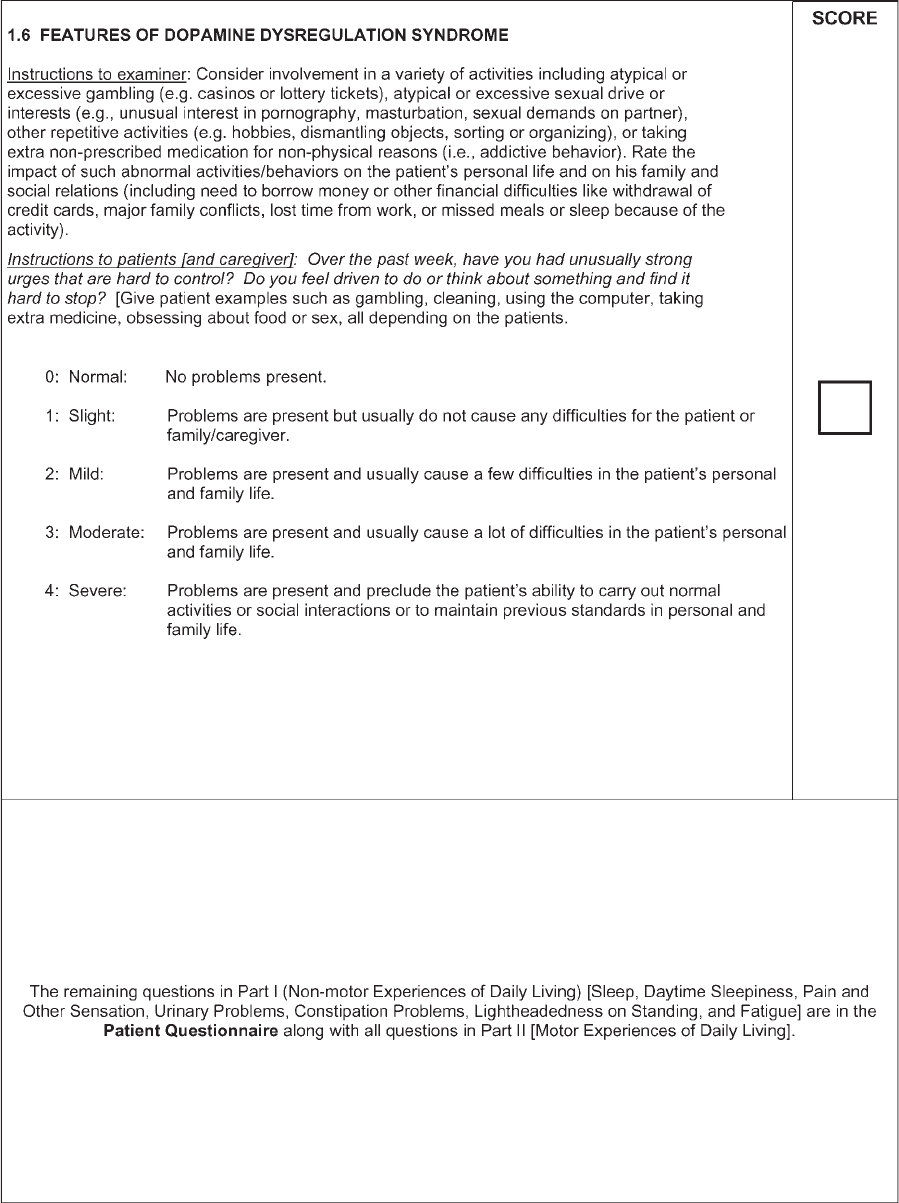
been both clarified and modified. Part I concerns ‘‘non-
motor experiences of daily living,’’ Part II concerns
‘‘motor exper iences of daily living ,’’ Part III is retained
as the ‘‘motor examination,’’ and Part IV concerns
‘‘motor complications.’’ Several questions from Part I
and all questions from Part II have been designed to
be amenable to a patient/caregiver questionnaire format
and therefore can be completed without the investiga-
tor’s input. For the remaining Part I questions that deal
with complex behaviors and all questio ns in Part IV
that deal with motor fluctuations and dyskinesias, the
investigator is required to conduct the interview. Part
III retains the objective assessments of parkinsonism,
but all tasks now have specific instructions. Rater
involvement time for administering the MDS-UPDRS
is estimated to require less than 10 min for the inter-
view items of Part I, 15 min for Part III, and 5 min for
Part IV, resulting in an equivalent rater time invest-
ment to the original scale and meeting the 30-min
goal. The remaining questionnaire items are answered
by the patient or caregiver and, other than supervision,
do not involve rater time. Whereas the detailed assess-
ments still prioritize the motor aspects of PD, the
screening questions on nonmotor elements are designed
to capture both the presence and severity of clinically
pertinent problems in this domain.
Each question is anchored with five responses that
are linked to commonly accepted clinical terms: 0 5
normal, 1 5 slight, 2 5 mild, 3 5 moderate, and 4 5
severe. After each clinical descriptor, a short text fol-
lows, which describes the criteria for each response.
Whereas each response is tailored to the question, the
progression of disability or impairment is based on a
consistent infrastructure. ‘‘Slight’’ (1) refers to symp-
toms/signs with sufficiently low frequency or intensity
to cause no impact on function; ‘‘mild’’ (2) refers to
symptoms/signs of frequency or intensity sufficient to
cause a modest impact on function; ‘‘moderate’’ (3) refers
to symptoms/signs sufficiently frequent or intense to
impact considerably, but not prevent, function; ‘‘severe’’
(4) refers to symptoms/signs that prevent functi on.
The full MDS-UPDRS contains questions/evalua-
tions (Table 1), divided across Part I (13), Part II (13),
Part III (33 scores based on 18 items, several with
right, left or other body distribution scores), and Part
IV (6). The MD S-UPDRS rates 65 items in comparison
2130 C.G. GOETZ ET AL.
Movement Disorders, Vol. 23, No. 15, 2008

TABLE 1. Conceptual mapping of items and scores from the original UPDRS to the MDS-UPDRS
MDS-UPDRS item Original UPDRS item
General concepts for mapping ratings from the original UPDRS to
MDS-UPDRS (UPDRS?MDS-UPDRS)
Part I In the MDS-UPDRS, the conceptual construct focuses on the impact
rather than the presence of symptoms, and whereas there is a general
parallelism between UPDRS and MDS-UPDRS, this emphasis needs
to be considered at all times by the rater and/or patient.
Cognitive impairment Intellectual impairment General conceptual comparison, although the emphasis is different in the
two versions: 0?0; 1?2 (option 1 on MDS-UPDRS is new and not
captured in original scale); 2?3; 3?4; 4?4
Hallucinations and psychosis Though disorder 0?0; 1?0 (vivid dreams not part of this question in MDS-UPDRS);
2?1or2;3?3; 4?4
Depressed mood Depression General conceptual comparison, although the emphasis is different in the
two versions: 0?0; 1?1; 2?2; 3?3; 4?4
Anxious mood
a
New item: No comparison
Apathy Motivation/initiative General conceptual comparison, although the emphasis is different in the
two versions: 0?0; 1?1; 2?2; 3?3; 4?4
Features of dopamine
dysregulation syndrome
a
New item: No comparison
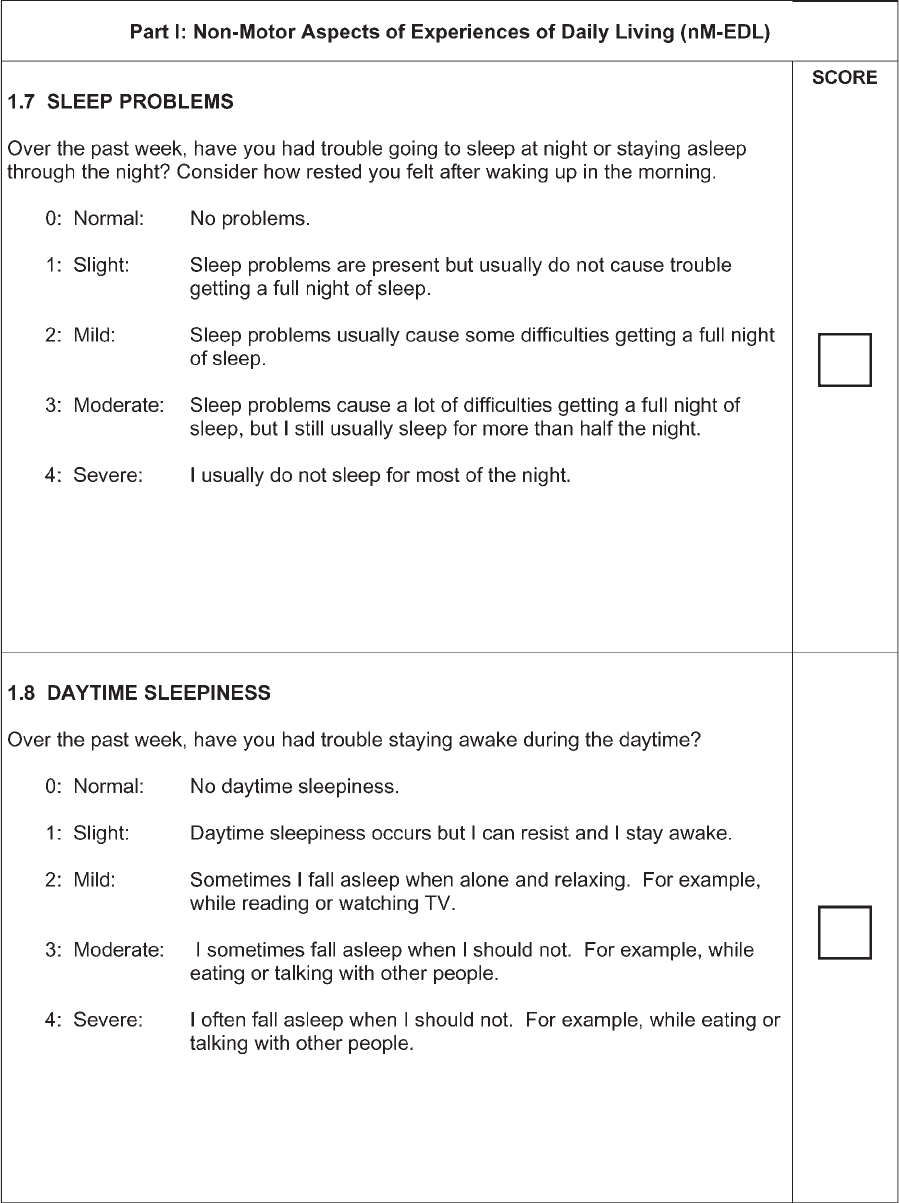
Nighttime sleep problems
a
Sleep disturbances 0?0; 1 on UPDRS could be 0 (if the patient had only daytime
sleepiness) or any of the available ratings on the MDS-UPDRS
Daytime sleepiness
a
Sleep disturbances 0?0; 1 on UPDRS could be 0 (if the patient had only nighttime sleep
problems) or any of the available ratings on the MDS-UPDRS
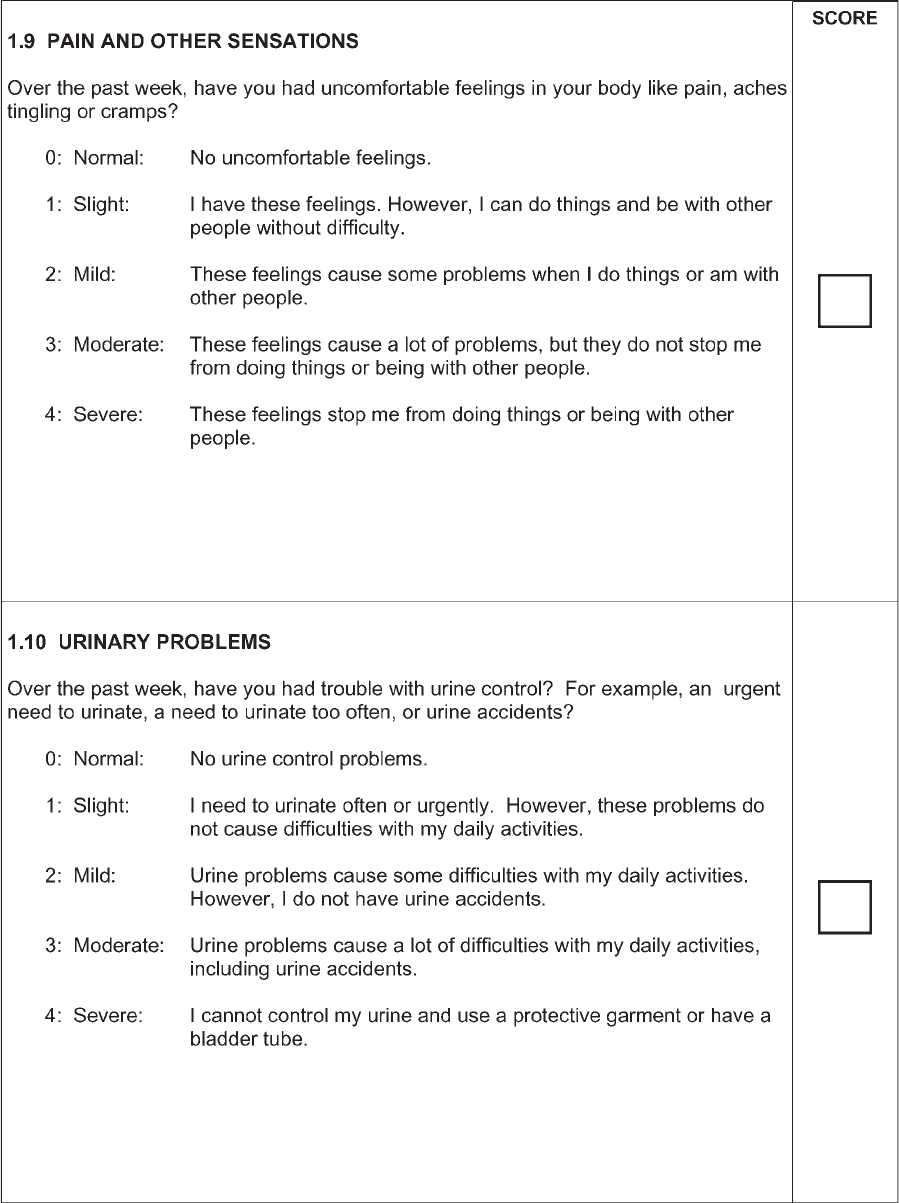
Pain and other sensations Sensory complaints
related to parkinsonism
General conceptual comparison, although the emphasis is different in
the two versions: 0?0; 1?1; 2?2; 3?3; 4?4
Urinary problems
a
New item: No comparison
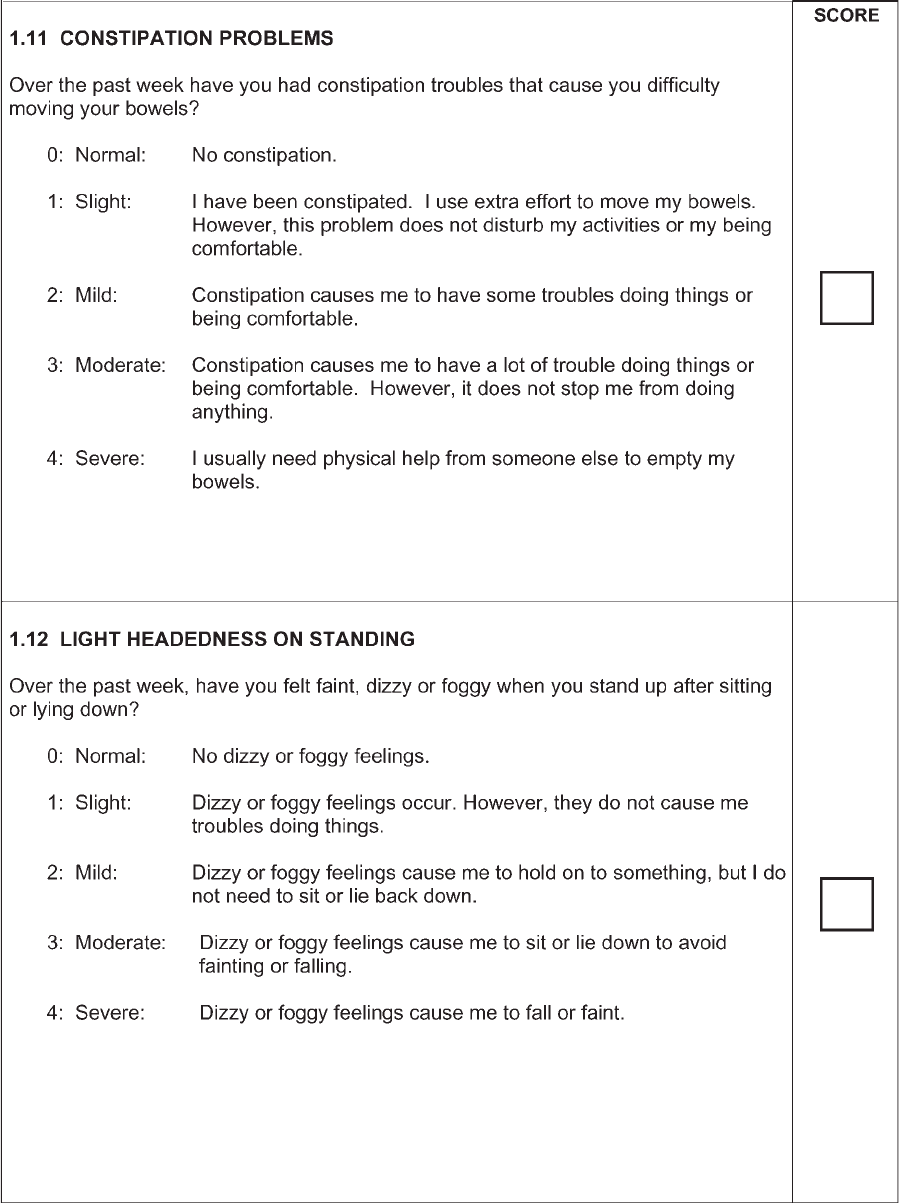
Constipation problems
a
New item: No comparison
Lightheadedness on standing
a
Symptomatic orthostasis 0?0; 1?1,2,3,4 depending on severity
Fatigue
a
New item: No comparison
Part II As in Part I, for the MDS-UPDRS, the conceptual construct focuses on
the impact rather than the presence of symptoms, and whereas there
is a general parallel between UPDRS and MDS-UPDRS, this
emphasis needs to be considered at all times by the rater and/or
patient.
Speech Speech 0?0; 1?1; 2?2, 3?3, 4?4
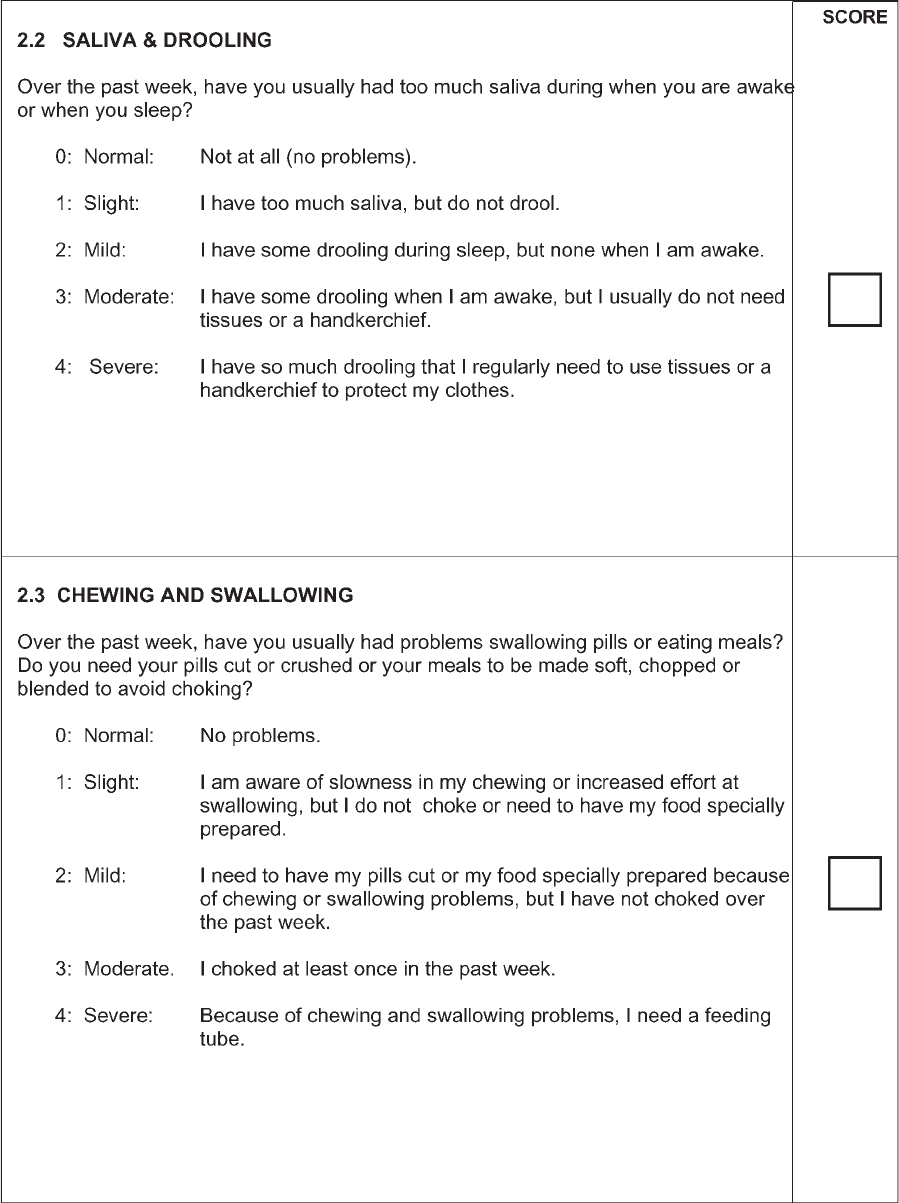
Salivation and drooling Salivation 0?0; 1?2 (option 1 on MDS-UPDRS new and not captured in original
scale; 2?3; 3?3; 4?4
Chewing and swallowing Swallowing General conceptual comparison, although the emphasis is different in the
two versions: 0?0; 1?3 (options 1 and 2 on MDS-UPDRS are new
and not captured well by the original scale); 2?3; 3?2; 4?4
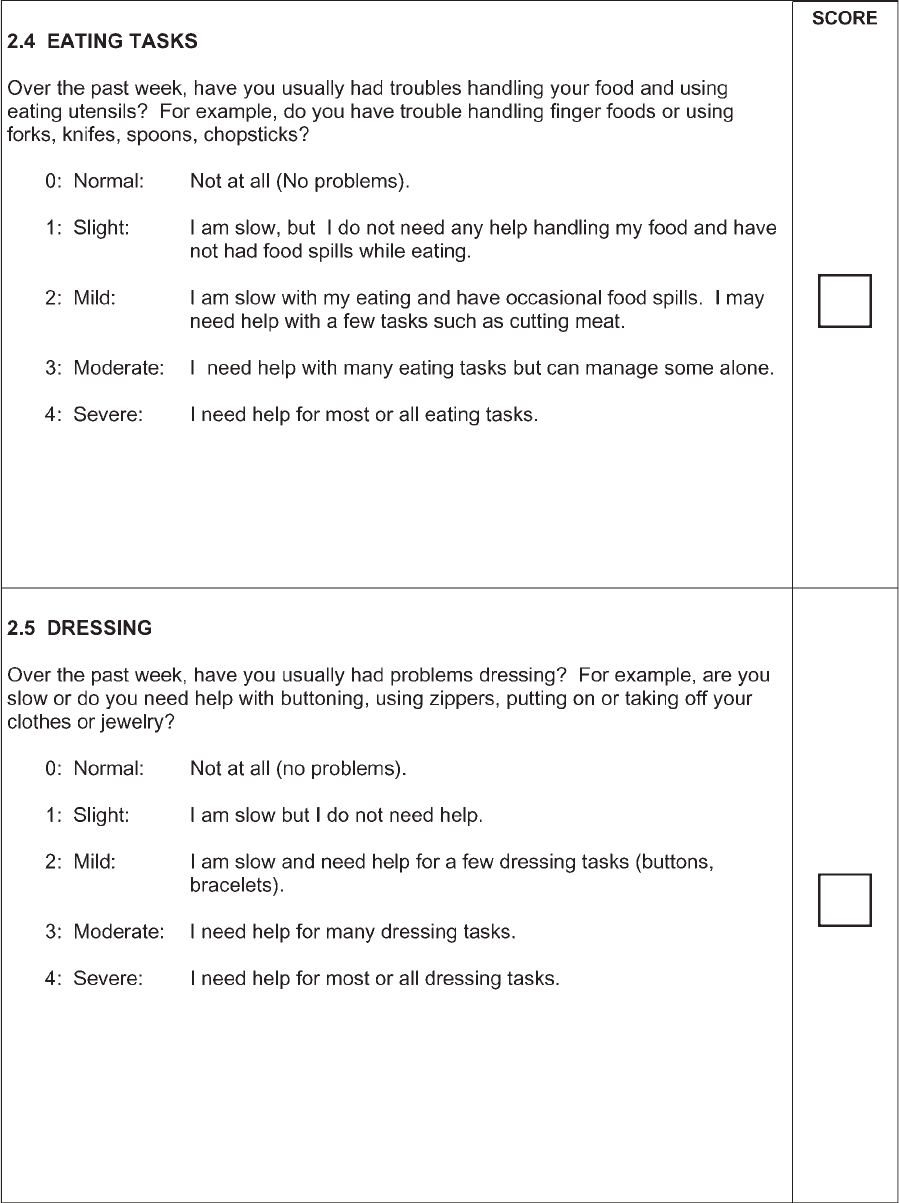
Eating tasks Cutting food and
handling utensils
0?0; 1?1; 2?2; 3?3; 4?4
Dressing Dressing 0?0; 1?1; 2?2; 3?3; 4?4
Hygiene Hygiene Although MDS-UPDRS focuses on all tasks and does not limit questions
to tasks mentioned in UPDRS, general parallelism exists for the two:
0?0; 1?1; 2?2; 3?3; 4?4
Handwriting Handwriting The MDS-UPDRS emphasizes clarity of writing, not size, but a general
parallelism exists for the two scales: 0?0; 1?1; 2?1; 3?2or3;
4?4
Doing hobbies and
other activities
a
New item: No comparison
Turning in bed Turning in bed and
adjusting bed clothes
The MDS-UPDRS emphasizes regularity of help needed, but a general
parallelism exists for the two scales: 0?0; 1?1; 2?2or3;3?3or
4; 4?4
Tremor Tremor The MDS-UPDRS emphasizes interference from tremor, but a general
parallelism exists for the two scales: 0?0; 1?1; 2?2; 3?3; 4?4
Getting out of bed, car,
or deep chair
a
New item: No comparison
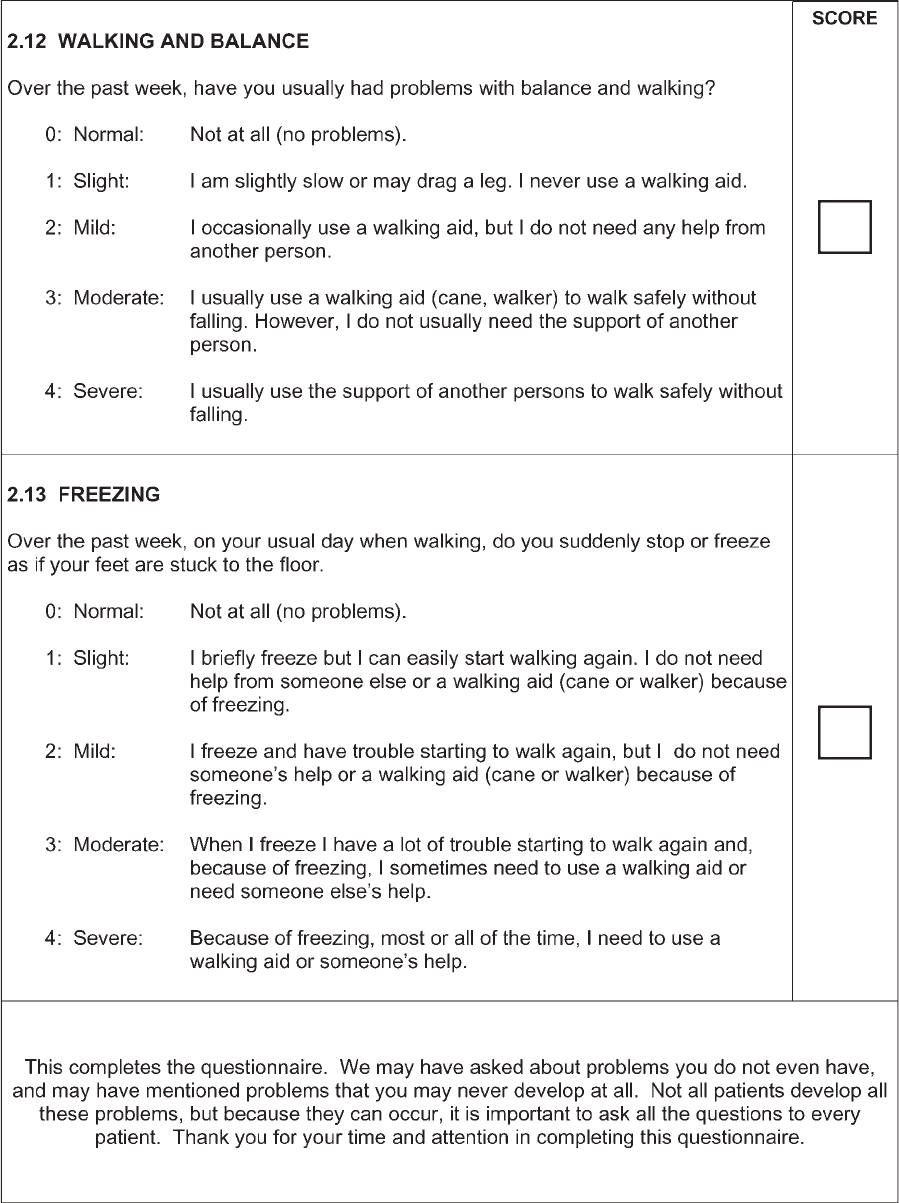
Walking and balance Walking 0?0; 1?1; 2?1or2;3?3or4;4?4
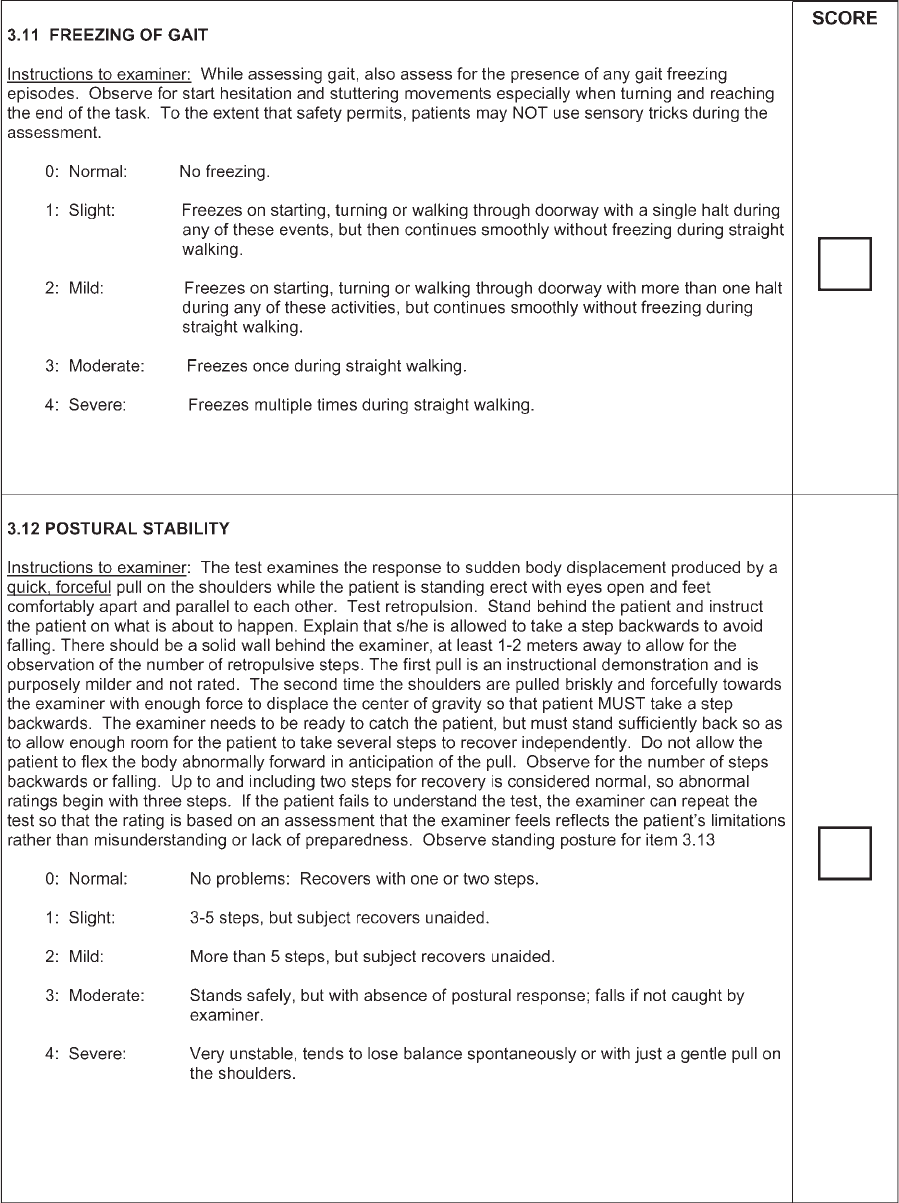
Freezing Freezing when walking Conceptually, the focus of the MDS-UPDRS is different from the
UPDRS because the need for assistance is emphasized in the MDS-
UPDRS rather than the consequence (falls) that will depend on
availability of help. Only partial parallelism can be drawn on this
question: 0?0; 1?1; 2?2,3,or 4; 2?2, 3, or 4; 4?2, 3, or 4
Falls This item is not part of the MDS-UPDRS because it is not a normal
‘‘experience of daily living.’’ Falling is assessed in Part III
2131MDS-UPDRS: CLINIMETRIC ASSESSMENT
Movement Disorders, Vol. 23, No. 15, 2008

to 55 on the original UPDRS, 48 that had 0 to 4
options and 7 with yes/no responses.
Nine new items in the MDS-UPDRS were not cap-
tured in any form on the original scale: anxious mood,
dopamine dysregulation syndrome, urinary problems,
constipation, fatigue, doing hobbies, getting in and out
of bed, toe tapping, and freezing (objective rating).
Lightheadedness was assessed in the original UPDRS
as present or absent, but in the MDS-UPDRS, the
symptom is assessed with the 0 to 4 rating system.
Nighttime sleep problems and daytime sleepiness are
assessed in the MDS-UPDRS and the yes/no sleep dis-
TABLE 1. (Continued )
MDS-UPDRS item Original UPDRS item
General concepts for mapping ratings from the original UPDRS to
MDS-UPDRS (UPDRS?MDS-UPDRS)
Part III
Speech Speech 0?0; 1?1; 2?2; 3?3or4;4?4
Facial expression Facial expression 0?0; 1?1; 2?2; 3?3; 4?4
Rigidity of neck and
four extremities
b
Rigidity Conceptually, the focus of the question has been changed to emphasize
resistance to passive movement with greater clarity. Partial parallelism can
be suggested: 0?0; 1?1; 2?2; 3?2; 4?3; 4 rating on the MDS-UPDRS
is not captured by the original UPDRS
Finger taps
b
Finger taps The original UPDRS had descriptors (mild, moderate, severe), that fit better
with the current designations of slight, mild and moderate, creating
difficulties with a direct parallelism, but the task descriptions allow
parallelism: 0?0; 1?1or2;2?2or3;3?3; 4?4
Hand movements
b
Hand movements See ‘‘finger taps’’ for explanation: 0?0; 1?1or2;2?2or3;3?3; 4?4
Pronation/supination
b
Pronation/supination See ‘‘finger taps’’ for explanation: 0?0; 1?1or2;2?2or3;3?3; 4?4
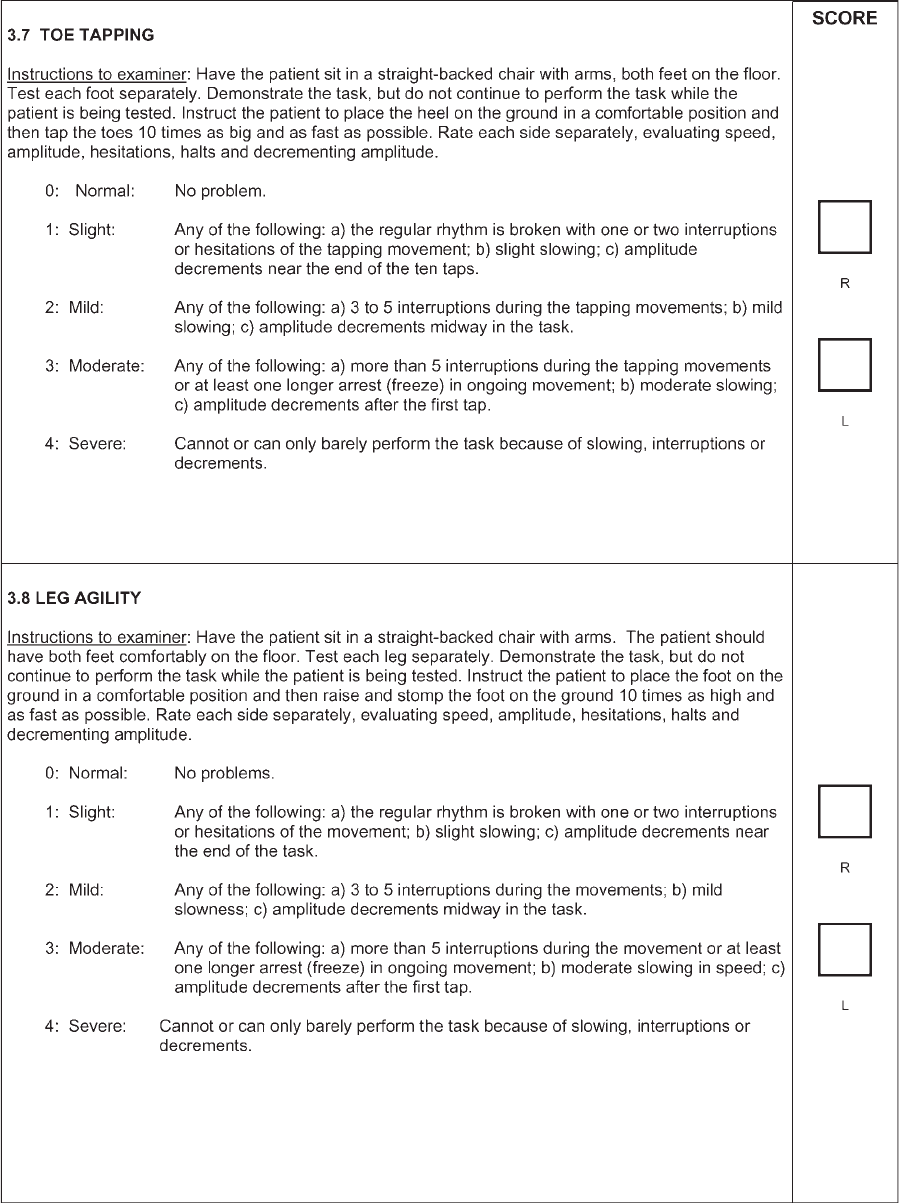
Toe tapping
b
New item; no comparison
Leg agility
b
Leg agility See ‘‘finger taps’’ for explanation: 0?0; 1?1or2;2?2or3;3?3; 4?4
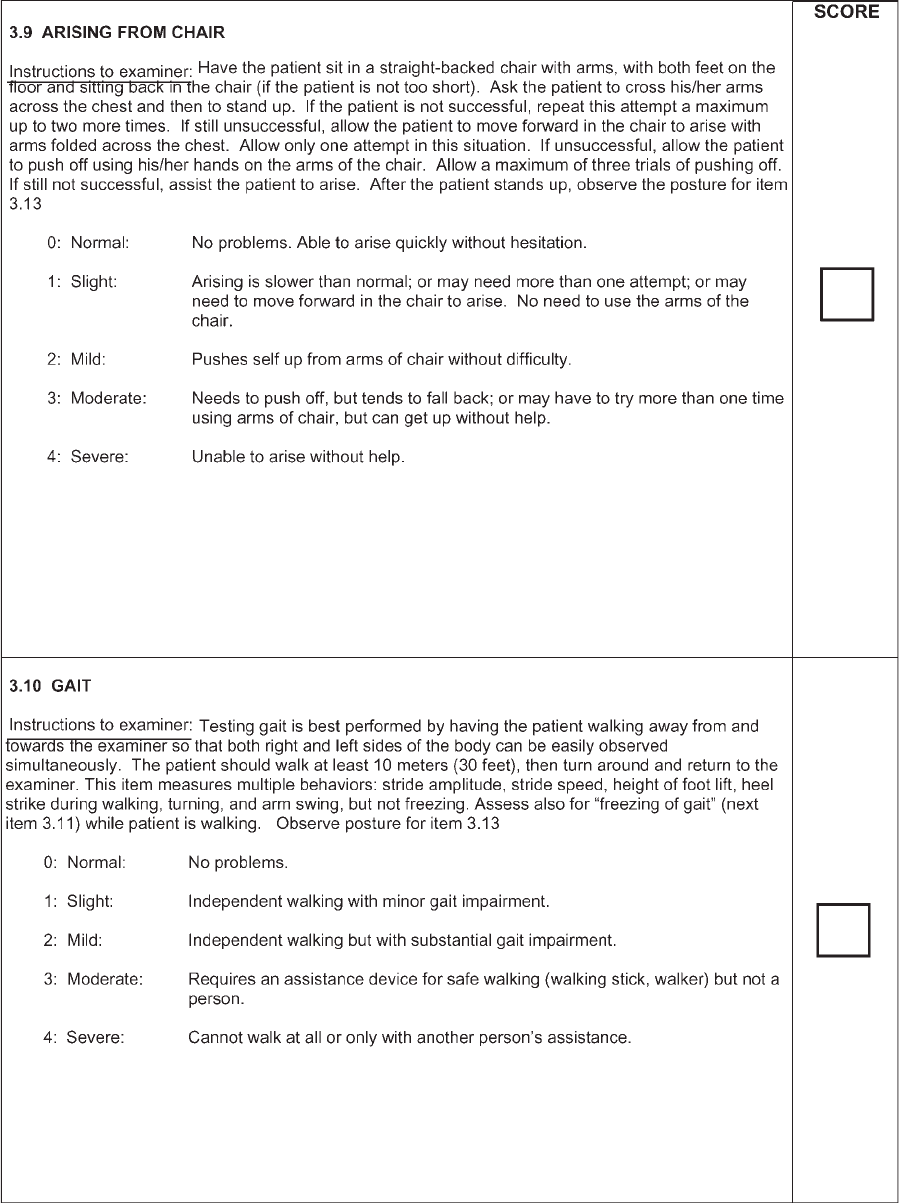
Arising from chair Arising from chair 0?0; 1?1; 2?2; 3?3; 4?4
Gait Gait 0?0; 1?1; 2?2; 3?3or4;4?4
Freezing of gait
a
New item: no comparison from original scale
Postural stability Postural stability 0?0; 1?1or2;2?3; 3?4; 4?4
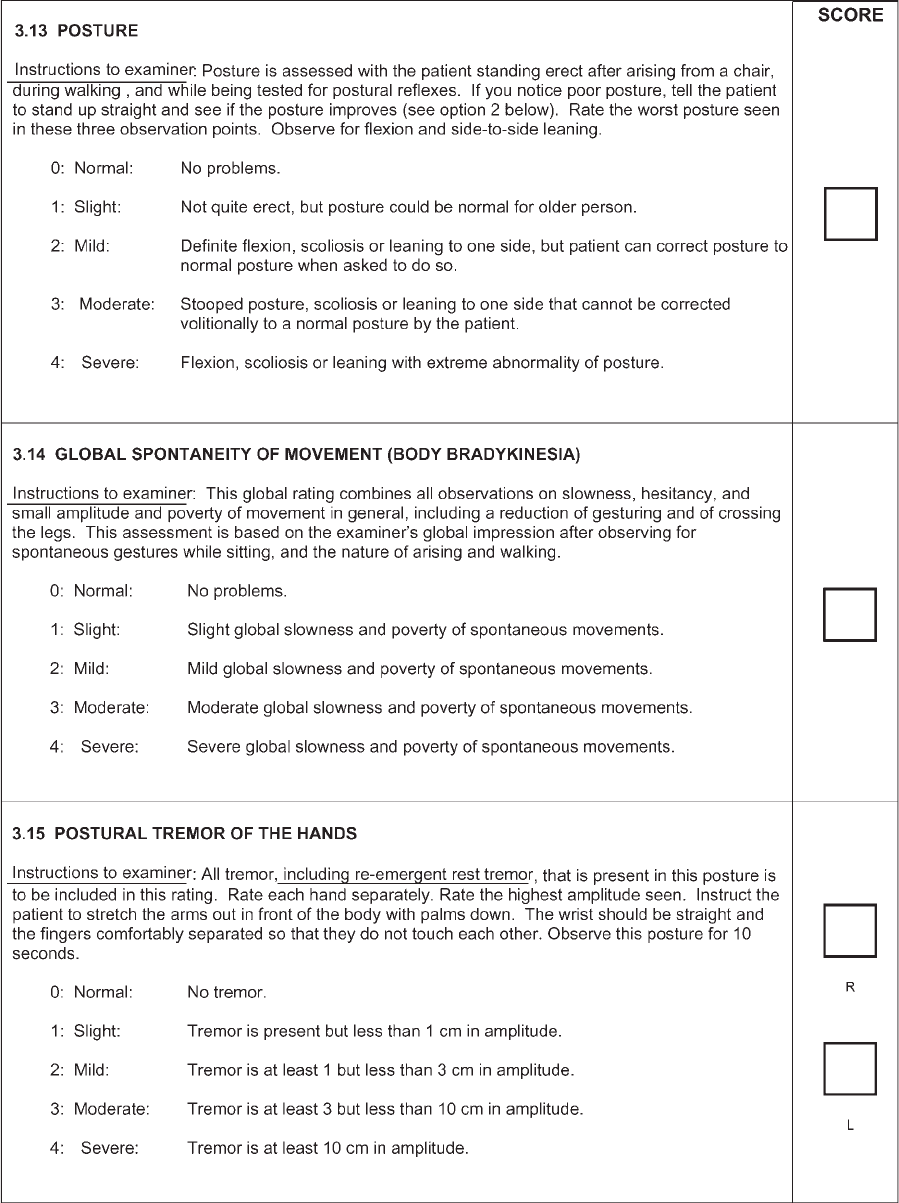
Posture Posture 0?0; 1?1; 2?2or3;3?4; 4?4
Global spontaneity of
movement
Body bradykinesia 0?0; 1?1; 2?2; 3?3; 4?4
Postural tremor of hands
a,b
Action/postural
tremor
The MDS-UPDRS separates these two forms of tremor and focuses only on
amplitude, so there is no parallelism between the original and new versions
Re-emergent tremor is rated as part of postural tremor (see Discussion)
Kinetic tremor of hands
a,b
Action/postural
tremor
Rest tremor amplitude
b
The MDS-UPDRS separates two features of rest tremor (amplitude and
consistency), so there is no parallelism between the original and new versions
Constancy of rest tremor
a
New item: no comparison. Tremor consistency was considered in original
UPDRS but combined with amplitude, making the assessment ambiguous
Part IV
Time spent with
dyskinesia
Dyskinesia duration 0?0; 1?1; 2?2; 3?3; 4?4
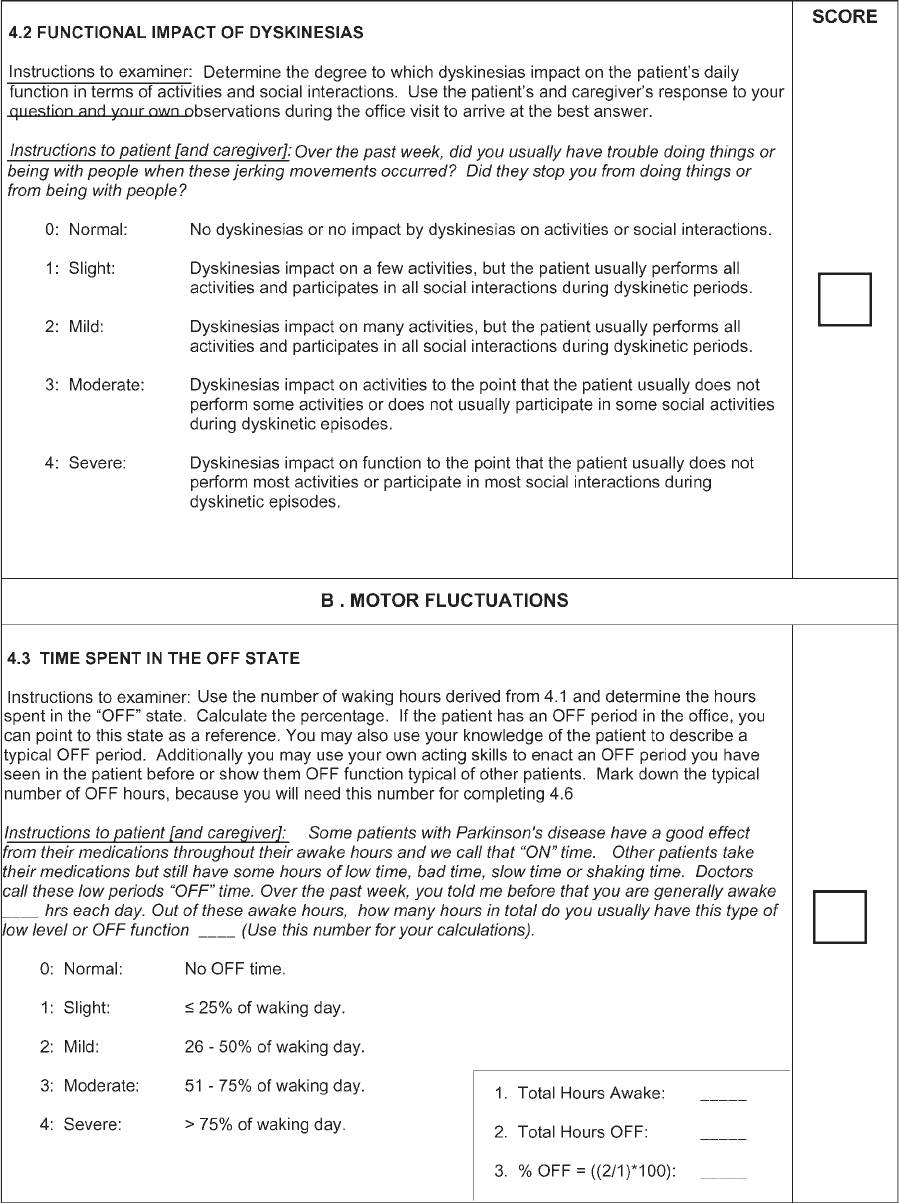
Functional impact
of dyskinesias
MDS-UPDRS provides written anchors whereas the UPDRS uses only ‘‘mild,
moderate, severe, marked.’’ 0?0; 1?2 (option 1 on MDS-UPDRS new and
not captured in original scale); 2?3; 3?4; 4?4
Time spent in the
OFF state
Off duration 0?0; 1?1; 2?2; 3?3; 4?4
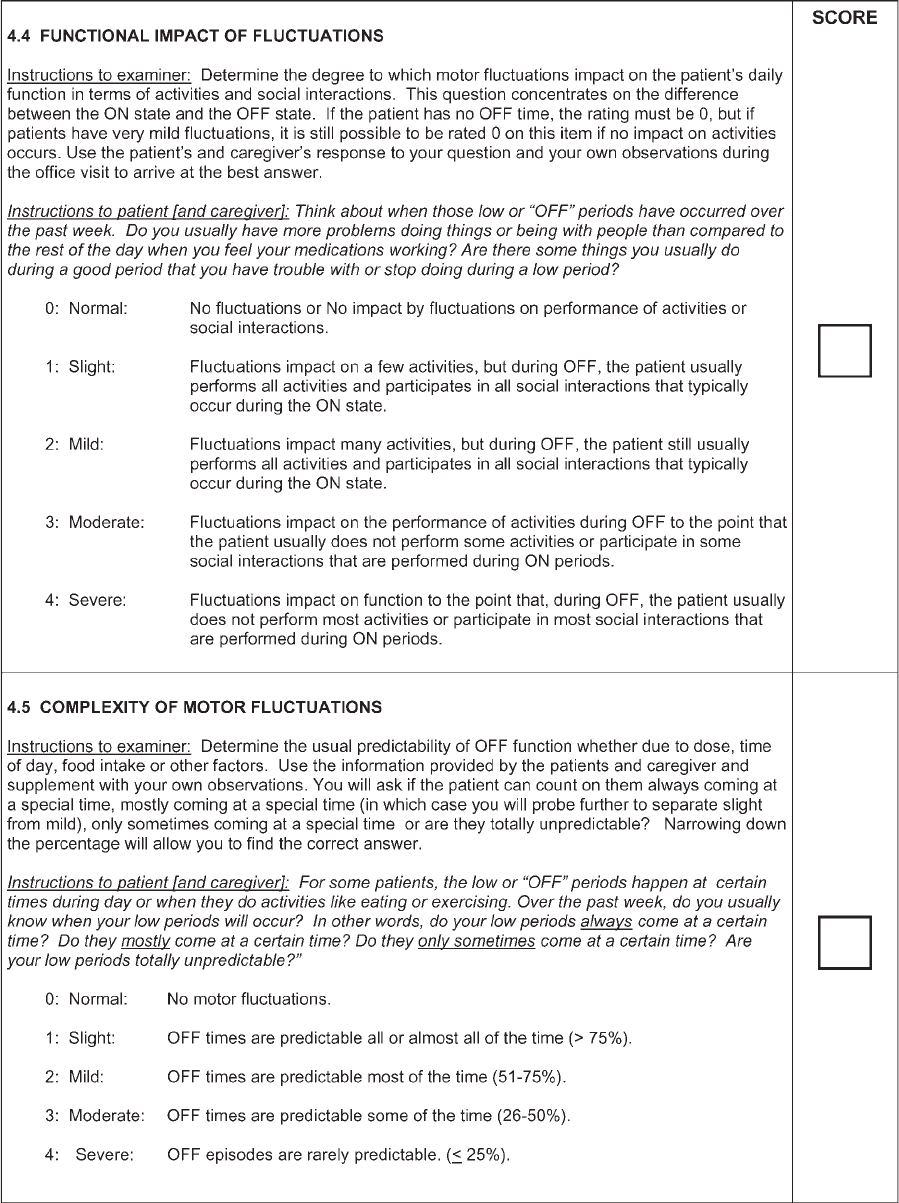
Functional impact
of fluctuations
New item: no comparison. Written to run in parallel with Function impact of
dyskinesias
Complexity of motor
fluctuations
a
Offs predictable
(yes/no) Offs
unpredictable
(yes/no) Offs
sudden (yes/no)
MDS-UPDRS consolidates concepts covered by several yes/no questions on
UPDRS. There is no simple mapping for this reason
Painful OFF-state dystonia Presence of early
morning dystonia
(yes/no)
0?0; 1?1, 2, 3, or 4
Many items have shifted emphasis with the MDS-UPDRS, but this guide shows the general concept behind the two scoring systems and can be
used as a reference. The mapping table is a guide and not recommended as an automatic transfer for scores from one scale to the other. Gray box
marks items covered by patient/caregiver questionnaire without direct input from the investigator.
a
Domains not previously assessed with 0 to 5 ratings.
b
Items with right and left measurements.
2132 C.G. GOETZ ET AL.
Movement Disorders, Vol. 23, No. 15, 2008
turbances option is replaced from the original UPDRS.
The question on Complexity of Motor Fluctuations in
the MDS-UPDRS merges the three yes/no questions
related to predicable, unpredictable, and sudden OFF
period from the UPDRS. In regards to tremor, the orig-
inal Action/Postural Tremor question has been divided
into two questions focusing on each component of
tremor separ ately. For rest tremor, whereas the UPDRS
combined amplitude and constancy of tremor into its
descriptors, on the MDS-UPDRS the severity ratings
for each body part concern only the amplitude and a
separate question rates the constancy.
Direct item-to-item mapping from the original
UPDRS to the MDS-UPDRS was not envisioned to be
possible, because the two scales were not conceptually
identical. Nonetheless, because the new version was
directly based on the original scale, a number of paral-
lels and guidelines were utilized in the construction of
the MDS-UPDRS. For some questions, the insertion of
slight/mild/moderate/severe was sufficient to realign
the rating options. In some cases, however, because the
original scale often used ‘‘mild/moderate/severe/
marked’’ the former rating of 1 (mild) now could be
separated into two choices (slight 1 or mild 2) in the
MDS-UPDRS. In such cases, the former moderate
scores (2) advanced to 3 in the MDS-UPDRS and the
former severe (3) and marked (4) were collapsed into
one option (severe 4) in the MDS-UPDRS. In other
cases, adjustments in the mid-ranges (2 and 3) were
felt to be necessary in order to maintain a consistent
conceptual framework of slight/mild/moderate/severe
in the MDS-UPDRS. This decision to shift from mild/
moderate/severe/marked to slight/mild/moderate/severe
as the scale’s clinical construct was anchored in two
concepts: first, that many clinical trials focus on early
PD where change among scores of normal, slight, and
mild problem s are important to document; and, second,
that at the high range of impairment or disability (for-
merly severe and marked), functional differences may
not be clinical ly relevant. Another conceptual anchor
of the MDS-UPDRS, especially apparent in Parts I, II,
and IV was the progressing disability from none (0) to
a perception of the problem without interference (slight
1), to interference with isolated activity (mild 2), to in-
terference with normal activity (moderate 3), to preclu-
sion of normal activity (severe 4). This process was
not utilized consistently in the original UPDRS,
although parallels could be constructed between the
two versions in many cases. Yes/no questions from the
original Part IV were reformatted and refined to fit the
0 to 4 rating format of the rest of the scale in the
MDS-UPDRS, so that a partial parallelism between the
two versions could be mapped. New items that
assessed features not assessed in the original UPDRS
could not have any mapping possibility from the origi-
nal scale. With these caveats, general mapping patterns
between the two scales were outlined to allow a guide
to raters making the transition between the UPDRS
and the MDS-UPDRS, but were not constructed with
the aim of allowing automatic substitution (Table 1).
As part of the clinimetric plan, however, a review of
score ranges for each part of the MDS-UPDRS was
planned to be tested against the original version (see
below).
ON and OFF definitions are provided to ensure uni-
formity among raters and the score sheet documents the
ON/OFF status associated with the Part III assessment.
For Parts I and II, the official scale will not separate ON
from OFF, but, for special studies, the same questions can
be asked separately for ON or OFF periods. Throughout
the MDS-UPDRS, specific instructions are provided to
enhance a uniform application. Finally, questions have
been written to be culturally sensitive and applicable to
patients of different ethnic and social backgrounds.
As an ongoing process, at the end of the MDS-
UPDRS, clinicians and researchers are directed to an
Appendix of Additional Scales. This portion of the
MDS-UPDRS is not considered a static document, but,
instead, it will be updated as deemed appropriate by
the MDS Task Force of Rating Scales for PD (see
Supp. Info. Appendix 1 for listing of scales). This ap-
pendix is designed to direct clinicians and research
investigators to scales that cover in greater detail the
components of the MDS-UPDRS that are only assessed
with single items. The Task Force has previously pub-
lished assessments of scales for depression and psychosis,
and others are planned.
5,6
These assessments use a stand-
ard set of criteria to establish Recommended and Sug-
gested scales in an effort to encourage reporting in a con-
sistent manner and to facilitate comparisons among dif-
ferent reports. For items that have not had official Task
Force reports, the subcommittee of the MDS-UPDRS
dedicated to the Appendix has reviewed scales using the
same criteria (Cristina Sampaio, chairperson).
CLINIMETRIC TESTING PROGRAM
Methods
Based on successful prelimi nary testing,
4
the MDS-
UPDRS validation program was designed to test the
scale’s intrinsic attributes, including internal consis-
tency, factor structure, differential item functioning,
and its comparability with the original UPDRS.
2133MDS-UPDRS: CLINIMETRIC ASSESSMENT
Movement Disorders, Vol. 23, No. 15, 2008
We recruited movement disorder specialists and
experienced study coordinators to examine PD patients
with both scales. Special attention was focused on
recruitment of diverse race/ethnicity representations.
Native English speakers (both raters and patients) par-
ticipated. To complete separate clinimetric analyses
within each racial/ethnic group, a minimal sample size
of 650 per racial/ethnic group was desirable assuming
that at least 10 observations per item were required.
7
After obtaining individual IRB approval, each partici-
pating site recruited patients to under go both the
UPDRS and MDS-UPDRS in a single setting. Partici-
pation was based on a commitment to rate between 10
and 20 subjects who covered the range of mild to
severe PD, based on clinical judgment. Scores were
sent to a central database electronically and verified
for completeness. Queries were resolved between the
statistical center and individual raters, and once com-
pleted, a given case was entered into the full data set
and double-checked for accuracy.
Statistical Analyses
To describe patient demographics, we computed
means, standard deviations, and ranges. To assess rela-
tionships between the new and original version, we com-
puted Pearson’s correlations between the MDS-UPDRS
and the UPDRS for total score and for each part. Corre-
lations were computed to assess the relationships among
the parts of the MDS-UPDRS. As a measure of internal
consistency (reliability), Cronbach’s alpha was calculated
for each part. Further, floor and ceiling effects were
examined by calculating the percentage of lowest and
highest possible scores for each part. Mplus v. 4.21
8
was
used for the factor analysis using polychoric correlations
because of the ordinal nature of the data.
The factor analysis was run in two parts. An explor-
atory factor analysis (EFA), informed by eigenvalues
and Scree plots, was used to determine the number of
factors that best represents the data. A factor loading
cutoff of 0.40 was used to determine those items to
retain in a factor. As a second step, a confirmatory fac-
tor analysis (CFA) was used in the assessment of
dimensionality, with a comparative fit index (CFI) ‡
0.90 defined as an acceptable fit. If the CFI was less
than 0.90, each factor was examined to identify poorly
behaved items, i.e., those with high loadings on more
than one factor.
9
Based on the review of the items and
the model fit statistics, additional EFAs and CFAs
were run. The process was repeated until the most par-
simonious model was found with a CFI ‡ 0.90. We
assessed the entire scale as a single factor structure,
each part as a separate set of factors, and all combina-
tions of parts.
Results
Patient Sample
A total of 877 native English-speaking PD patients
were examined with the UPDRS and MDS-UPDRS
(560 men and 317 women). There were 682 non-Lat-
ino Caucasians and 195 (22%) of other race/ethnicity,
specifically 49 African Americans, 87 Latinos, 1 native
Hawaiian, 43 Asians, and 15 with other race/ethnic-
ities. All Hoehn and Yahr stages were represented, with
the majority of patients being stages II (stage I 5 63,
stage II 5 467, stage III 5 174, stage IV 5 109, stage
V 5 53, missing 11). The mean age of the cohort was
68.2 years (SD: 10.8; range: 31–98), and the mean PD
duration was 8.3 years (SD: 6.7; range: 0–40 years).
Fifty-seven patients were not treated with antiparkinso-
nian medications. A total of 685 patients were treated
with levodopa in combination with another symptomatic
treatment for PD, 115 patients were on symptomatic
therapy without levodopa, 5 patients were on levodopa
alone, and 15 had missing treatment information. Motor
fluctuations were observed in 483 patients and 304
patients had dyskinesia. A total of 723 were examined
in the ON state and 99 in the OFF state, while ON/OFF
information was not recorded for 55 patients.
Rater Sample
There were 69 raters from 39 English-speaking treat-
ment centers (USA, 32; Canada, 2; UK, 5) who eval-
uated patients with the original and MDS-UPDRS
instruments (see listing at end of manuscript). All
raters were physicians or nurse coordinators regularly
working with PD patients, regularly using the UPDRS
for clinical care or research purposes, and all were
recruited through the MDS. Raters were instructed to
perform the UPDRS in their standard manner and to
use the MDS-UPDRS following the instructions em-
bedded in the new scale for each item. The choice of
patients was left to the raters, but emphasis was placed
by the Task Force team on recruitment of a maximal
number of patients who were of a race/ethnicity other
than non-Latino Caucasian with a full breadth of Hoehn
and Yahr stages. The mean number of cases subm itted by
each rater was 11.4 (SD: 8.2; range: 1–29).
Clinimetric Profile of MDS-UPDRS
Internal consistency was computed for each of the
MDS-UPDRS parts [Part I (13 item s), alpha 5 0.79;
2134 C.G. GOETZ ET AL.
Movement Disorders, Vol. 23, No. 15, 2008

Part II (13 items), alpha 5 0.90; Part III (33 items),
alpha 5 0. 93; Part IV (6 items), alpha 5 0.79]. Mean
scores (SD) for each part were: Part I: 11.5 (7.0); Part
II: 16.0 (10.0); Part III: 36.8 (18.4); Part IV: 4.0 (4.2).
The distributions of the total scores in the MDS-
UPDRS and original UPDRS were similar: UPDRS
mean: 61.0 (SD: 30.3), covering 55 items; MDS-
UPDRS mean: 68.4 (SD: 32.8), covering 65 items. The
MDS-UPDRS showed strong concurrent validity based
on high correlations between the two scales (total score
r 5 0.96), as well as between the individual parts of
the two scales: [Part I, r 5 0.76; Part II, r 5 0.92; Part
III, r 5 0.96; Part IV (sum of items 32–39 covering
dyskinesias and motor fluctuations on the UDPRS vs.
total Part IV from the MDS-UPDRS), r 5 0.89]. As a
measure of internal validity, correlations among the
MDS-UPDRS parts were examined. These analyses
confirmed that each part assesses a different aspect of
PD, with mos t parts, except Parts I and II, having rela-
tively low correlations (Parts I and II, r 5 0.67; Parts I
and III, r 5 0.43; Parts I and IV, r 5 0.39; Parts II
and IV, r 5 0.44; Parts III and IV, r 5 0.22). As
anticipated, Parts II and III that covered patient percep-
tions of motor function and the objective examination
were more highly correlated (r 5 0.66). Our analysis
for possib le floor and ceiling effects demonstrated a
low percentage of lowest and highest scores for Parts I
TABLE 2. Factor structures of the four
parts of the MDS-UPDRS
Factor Item Item factor loading
Part I: Nonmotor aspects of experiences of daily living (CFI 5 0.94,
RMSEA 5 0.06)
Factor 1 Percent variance 5 32.7
Daytime sleepiness 0.57
Sleep problems 0.40
Cognitive impairment 0.48
Pain and other sensations 0.48
Hallucinations and psychosis 0.40
Urinary problems 0.59
Constipation problems 0.49
Features of DDS 0.49
Light headedness on standing 0.45
Fatigue 0.54
Factor 2 Percent variance 5 9.8
Depressed mood 0.83
Anxious mood 0.66
Apathy 0.53
Part II: Motor aspects of experiences of daily living (CFI 5 0.95,
RMSEA 5 0.09)
Factor 1 Percent variance 5 53.0
Speech 0.79
Saliva and drooling 0.45
Chewing and swallowing 0.60
Handwriting 0.45
Doing hobbies and other activities 0.45
Factor 2 Percent variance 5 8.7
Eating tasks 0.68
Tremor 0.43
Factor 3 Percent variance 5 7.7
Dressing 0.64
Hygiene 0.64
Turning in bed 0.65
Getting out of bed 0.73
Walking and balance 0.82
Freezing 0.76
Part III: Motor examination (CFI 0.91, RMSEA 5 0.10)
Factor 1 Percent variance 5 36.8
Speech 0.59
Facial expression 0.53
Arising from chair 0.77
Gait 0.87
Freezing of gait 0.83
Postural stability 0.81
Posture 0.70
Global spontaneity of movement 0.64
Factor 2 Percent variance 5 15.1
Rest tremor amplitude, RUE 0.72
Rest tremor amplitude, LUE 0.71
Rest tremor amplitude, RLE 0.73
Rest tremor amplitude, LLE 0.71
Rest tremor amplitude, lip/jaw 0.59
Constancy of rest tremor 0.88
Factor 3 Percent variance 5 6.4
Rigidity, neck 0.67
Rigidity, RUE 0.73
Rigidity, LUE 0.74
Rigidity, RLE 0.80
Rigidity, LLE 0.81
Factor 4 Percent variance 5 6.1
Finger tapping, right hand 0.67
Hand movements, right hand 0.66
Pronation/supination, right 0.68
TABLE 2. (Continued )
Factor Item Item factor loading
Factor 5 Percent variance 5 4.8
Finger tapping, left hand 0.69
Hand movements, left hand 0.72
Pronation/supination movements, left 0.65
Factor 6 Percent variance 5 4.6
Postural tremor, right hand 0.66
Postural tremor, left hand 0.72
Kinetic tremor, right hand 0.81
Kinetic tremor, left hand 0.80
Factor 7 Percent variance 5 3.3
Toe tapping, right foot 0.65
Toe tapping, left foot 0.63
Leg agility, right leg 0.64
Leg agility, left leg 0.62
Part IV: Motor complications (CFI 5 1.0, RMSEA 5 0.05)
Factor 1 Percent variance 5 63.6
Time spent in the OFF state 0.87
Functional impact of fluctuations 0.84
Complexity of motor fluctuations 0.83
Painful OFF state dystonia 0.49
Factor 2 Percent variance 5 15.6
Time spent with dyskinesias 0.72
Functional impact of dyskinesias 0.94
CFI, comparative fit index; RMSEA, root mean square error of
approximation.
2135MDS-UPDRS: CLINIMETRIC ASSESSMENT
Movement Disorders, Vol. 23, No. 15, 2008
to III: Part I, lowest 0.1%/highest 0.8%; Part II, lowest
0.1%/highest 0.7%; Part III, lowest 0.1%/highest 0.2%.
In the case of Part IV, covering the presence and severity
of motor complications, there was an expected floor
effect, but no ceiling effect: lowest 36.7%/highest 0.1%.
(see Supp. Info. Appendix 2 for histograms of each part).
Factor Structure
Exploratory testing of the combined four parts of the
MDS-UPDRS did not identify a single factor structure
that could be confirmed (CFI 5 0.74). A factor struc-
ture combining Parts II and III was explored, but could
not be confirmed. Several items had salient loadings on
more than one factor and some items did not load on
any factor. A factor structure was also explored for the
combination of Parts II to IV. A factor structure with
12 factors was identified by the EFA; however, the CFI
was <0.90, too low to provide confirmation. As seen
earlier, several items had salient loadings on more than
one factor, and some items did not load on any factor.
These combined results preclude using a total MDS-
UPDRS score or scores based on combinations of parts.
We then analyzed the MDS-UPDRS parts individu-
ally (see Supp. Info. Appendix 3 for Scree plots). This
analysis and the confirmatory analysis identified a fac-
tor structure that was statistically consistent (CFI for
each part was >0.90) and clinically meaningful (Ta-
ble 2) for all parts. For Part I (CFI 5 0.94), two
factors were identified, one covering depression, anxi-
ety, and apathy and the other covering the other non-
motor functions (shared variance 5 42.5%). For Part II
(CFI 5 0.95), three factors were identified, one cover-
ing several fine motor functions, one covering tremor
and eating tasks, and one focusing on several large
motor functions (shared variance 5 69.4%). For Part
III (CFI 5 0.91), seven factors were identified: midline
function, rest tremor, rigidity, bradykinesia right upper
extremity, bradykinesia left upper extremity, postural
and kinetic tremors, and lower limb bradykinesia
(shared variance 77.1%). For Part IV (CFI 5 1.0), two
factors were identified, one focusing on fluctuations
including off-state dystonia and the other on dyskine-
sias (shared variance 5 79.2%). Intercorrelations
among factors for each part ranged from 0.04 to 0.71,
indicating both unique and shared information provided
by the different factors.
DISCUSSION
The MDS-UPDRS was designed to be more compre-
hensive than the ori ginal UPDRS, with new items
devoted to several nonmotor elements of PD.
3,4
The
choice of the new items was based on input from the
Task Force committee members, patient groups, and
MDS members. Based on the published critique of the
UPDRS,
3
the five-point range for each item was
retained, and clinical anchors of normal (0), slight (1),
mild (2), moderate (3), and severe (4) were added to
provide a consistency across item s. Importantly, the
MDS-UPDRS places greater emphasis on distinguish-
ing relatively mild impairments and disabilities,
drawing distinctions between slight and mild, whereas
former distinctions between severe and marked are
now collapsed into the severe rating (4). This decision
was anchored in the realities that clinical trials are
focusing increasingly on early disease, and functional
differences betwee n severe and marked impairments
from the original scale may not be clinically relevant.
As a result of this decision, for several items in the
MDS-UPDRS, moderate impairment and disability is
now rated as 3 instead of 2.
An important addition to the MDS-UPDRS is a set
of detailed instructions. Because the MDS-UDPRS is
envisioned to be the primary international rating scale
for PD clini cal care and research, an emphasis was
placed on clear and detailed descriptions of methods
for data acquisition. These are officially part of the
scale, so that international colleagues can perform rat-
ings in a systematic manner within and across centers.
The instructions are intended to standardize the method
of application of the scale so that the MDS-UPDRS
data are collected uniformly. The scale has not yet
been translated into non-English editions, and this
effort will start in 2008 through the MDS. A clinimet-
ric program for each language edition is planned and
the Task Force will offer statistical assistance.
The MDS-UPDRS involves participa tion by patients
and caregivers for the assessment of several nonmotor
and motor experiences of daily living. These questions
were written at seventh grade level and extensively
tested in patient focus groups. The question on fatigue
was included based on patient responses that this
symptom has a high impact on health-re lated quality of
life and was not otherwise captured in the scale.
Because cogni tive impairments frequently occur in
PD, the questionnaire was designed to be completed by
the patient alone, with the input of caregivers, or by
the caregiver alone, depending on patient/caregiver
preference.
Given the number of items to be assessed in the
MDS-UPDRS, the patient sample required for adequate
statistical analysis was large. We were, however, suc-
cessful in recruiting colleagues internationally from
2136 C.G. GOETZ ET AL.
Movement Disorders, Vol. 23, No. 15, 2008
English-speaking centers to help in this important
effort. These colleagues were able to identify and
examine PD patients across the spectrum of disabilities.
Investigators were asked to select a gamut of severities
among PD patients and to be attenti ve to obtaining di-
versity in gender and race/ethnicity. The distribution of
this data set in terms of disease severity, drug treatme nt,
gender, and ethnic balance should not be considered to be
representative of the investigators’ overall practice popu-
lation, because the composition reflects an effort to have
a clinically reasonable number of cases in different cate-
gories to test the clinimetric properties of the scale. None-
theless, the majority of patients were Hoehn and Yahr
stages II and III, a pattern seen in cross-sectional analyses
in early, mid, and late disease.
10
Based on the wide range
of severities sampled and the distribution of high and low
scores within each part, we are confident that the MDS-
UPDRS is not limited by floor or ceiling effects.
We placed special emphasis on the recruitment of
subjects of diverse race/ethnicity. Although we did not
achieve our goal of 650 per racial/ethnic group, we did
succeed in involving a much higher perc entage of sub-
jects of race/ethnicity other than non-Latino Caucasian
(N 5 195, 22% of our total sample) than the usual 0%
to 10% reported in clinical trials (personal communica-
tion, M. Schneider and C. Swearingen). Other than
non-Latino Caucasians, we did not have enough partic-
ipants in any one racial or ethnic group to conduct sta-
tistical analyses within any specific subgr oup. Efforts
to enhance diversity in clinical trials of PD is a focus
of US government funding,
11
and this program demon-
strates that PD investigators are able to excee d current
performance in clinical trials. To continue to enhance
this data set, the statistical center for the program (e-
tional ratings of the UPD RS vs. MDS-UPDRS for
those patients other than non-Latino Caucasians.
The clinimetric analysis supports the reliability and
validity of the MDS-UPDRS. It performs extremely
well in comparison with the original version with high
internal consistency for the entire scale as well as high
internal consistency on each part. In addition, even
though restructured, each part of the MDS-UPDRS
correlates highly with the corresponding part of the
original scale. The scaling modifications and item addi-
tions to the MDS-UPDRS provide new informat ion
while still capturing the features of PD from the origi-
nal scale. On the other hand, because the item
responses (0, 1, 2, 3, 4) have been substantially modi-
fied in terms of wording and concept, we cannot pro-
vide an algorithm with point-to-point or summary con-
version numbers.
The technique of factor analysis is a particularly
strong clinical/s tatistical method for scale evaluation,
because it tests whether items cluster and allows clini-
cians to determine if these clusters fall into compo-
nents that represent clinically relevant domains. Fur-
thermore, it allows statistical assessment of whether
the clusters correlate and thereby capture information
about the overall entity being studied, in this case, PD.
The MDS-UPDRS has excellent factor validity, and
the factor analysis confirms that the items cluster in
clinically pertinent domains. Because data are collected
using three different methods, some based exclusively
on patient or caregiver responses (questionnaire), some
based solely on the investigator’s assessments (motor
examination), and some with a combination (complex
behaviors and motor complications), we did not antici-
pate that the total score (combined Parts I–IV) would
likely be a recommended outcome. Although the high
correlation between the total scores on the original
UPDRS and MDS-UPDRS demonstrates that the two
scales are measuring the same overall entity of PD, the
MDS-UPDRS factor analysis confirmed that neither
the combined parts nor combinations based on differ-
ent acquisition methods have a stable factor structure.
However, when each part is considered separately, the
factor structures are both clinimetrically sound and
clinically pertinent. In this light, we recommend that
each of the parts (I–IV) should be reported separately
and not collapsed into a sing le ‘‘Total MDS-UPDRS’’
summary score.
Comparing the factor structure of Part III of the
MDS-UPDRS to published factor analyses of the origi-
nal UPDRS,
12
the MDS-UPDRS identifies lower limb
bradykinesia as a new factor, likely because of the
addition of toe tapping as a separate rating. Attention
was directed to separating postural from kinetic tremor,
and extensive discussion within the group focused on
the placement of ‘‘reemergent rest tremor.’’ Because
reemergent tremor interferes with the holding of
objects against gravity, this tremor was relegated to
postural tremor.
13
Despite these deliberations, the fac-
tor structure identified postural and kinetic tremors as a
single factor and the rest tremor was distinct from this
factor.
The final step in the clinimetric analysis will involve
an assessment of differential item function (DIF).
Although this type of analysis was never performed on
the original UPDRS, our large sample size and avail-
able statistical programs will allow this level of scru-
tiny for all items. DIF is defined as group differences
in item response, conditional on the state or trait
assessed. As an example, if in two groups defined by
2137MDS-UPDRS: CLINIMETRIC ASSESSMENT
Movement Disorders, Vol. 23, No. 15, 2008
gender, one rarely endorsed a high score for an item,
while the other often endorsed a high score, but the
two had similar scores on the overall part, this differ-
ence suggests that gender has an influence on the item
interpretations or responses. DIF may be due to group
differences in neurological burden, comp rehension, ad-
aptation, or bias. There are two types of DIF: uniform
and nonuniform. Uniform DIF is present when item
thresholds differ between the groups, but the slopes are
parallel on the item characteristic curves. Nonuniform
DIF is present when the two curves do not follow a
linear progression acro ss the rating options. The pres-
ence of either form of DIF would suggest that the item
in question does not perform the same in different
groups of the patient sample. We plan to examine three
patient characteristics for the DIF analysis in our study
sample: gender, race/ethnicity, and age. If DIF is identi-
fied, future modifications will be considered and tested
in subsequent phases of our clinimetric program (see
below). In this light, although we present the MDS-
UPDRS for immediate application in clinical settings,
we emphasize that the scale will continue to be evaluated
and that further refinements may develop in the future
based on additional and ongoing clinimetric analyses.
The Appendix of Additional Scales is officially part
of the MDS-UPDRS and directs clinicians and
researchers to scales that focus in more detail on areas
of disability that are considered as single-item ques-
tions on the MDS-UPDRS. The MDS Task Force on
Rating Scales in PD has initiated a number of critiques
of availab le scales dealing with different areas of dys-
function, and rankings of Recommended and Suggested
have been developed using predefined criteria.
5,6
The
results of these reports have been supplemented by
assessments by the MDS -UPDRS subcommit tee dedi-
cated to the Appendix and, as new clinimetric reports
on scales are publishe d and new scales are introduced,
the Appendix will be updated. Because the nonmotor
aspects of PD are an increasing focus of clinical deci-
sion-making and research, we recommend a uniform
selection of scales so that differ ent reports can be com-
pared with similar measures.
The next steps in the MDS-UPDRS program include
the non-English translations, testing the MDS-UDPRS
for responsivity to change over time, and analysis of
questions with DIF. The planned clinimetric program
leaves several additional projects available for investi-
gator-initiated research. Correlations between the
MDS-UPDRS and other scales such as quality of life
measures or global disease burden scales that are not
specific for PD are encouraged by the authors, but are
not part of this core program. Future clinical trials in
PD will tend to be of longer duration (often 5 years or
longer) as new therapies are tested to delay progression
post-levodopa administration. The long duration makes
it unlikely that the participant in a trial will have the
same rater at every visit. Thus, it is important that tem-
poral stability, sensitivity to change, and interrater reli-
ability be established in the MDS-UPDRS. To facilitate
interrater reliability, a Teaching Tape, modeled after
the one developed for the motor section of the original
UPDRS, is being developed.
14
The MDS-UPDRS is available on the MDS web site
(www.movementdisorders.org). Likewise, the Appen-
dix of Additional Scales is also available electronically
and will be updated through the MDS web site.
Acknowledgments: The MDS received unrestricted grants
for the development of the UPDRS revision program from
Boehringer-Ingelheim (USA), GlaxoSmithKline, and Pfizer,
Inc. The UK Parkinson’s Disease Society also provided sup-
port for assessment of subjects in the United Kingdom. Fund-
ing was also provided by the NINDS U01NS043127. We and
the MDS acknowledge the work of the following colleagues
whose data formed the core of the clinimetric comparison
between the UPDRS and MDS-UPDRS: Pinky Agarwal,
Saima Athar, Yvette Bordelan, Helen M. Bronte-Stewart,
Richard Camicioli, Kelvin Chou, Wendy Cole, Arif Dalvi,
Holly Delgado, Alan Diamond, Jeremy P. Dick, John Duda,
Rodger J. Elble, Carol Evans, Virgilio G. Evidente, Hubert
H. Fernandez, Susan Fox, Joseph H. Friedman, Robin D.
Fross, David Gallagher, Christopher G. Goetz, Deborah Hall,
Neal Hermanowicz, Vanessa Hinson, Stacy Horn, Howard
Hurtig, Un Jung Kang, Galit Kleiner-Fisman, Olga Klepit-
skaya, Katie Kompoliti, Eugene C. Lai, Maureen L. Leehey,
Iracema Leroi, Kelly E. Lyons, Terry McClain, Steven W.
Metzer, Janis Miyasaki, John C. Morgan, Martha Nance,
Joanne Nemeth, Rajesh Pahwa, Sotirios A. Parashos, Jay S.
Schneider, Anette Schrag, Kapil Sethi, Lisa M. Shulman,
Andrew Siderowf, Monty Silverdale, Tanya Simuni, Mark
Stacy, Matthew B. Stern, Robert Malcolm Stewart, Kelly
Sullivan, David M. Swope, Pettaruse M. Wadia, Richard W.
Walker, Ruth Walker, William J. Weiner, Jill Wiener, Jayne
Wilkinson, Joanna M. Wojcieszek, Summer Wolfrath, G.
Frederick Wooten, Allen Wu, Theresa A. Zesiewicz, Richard
M. Zweig.
Author Roles: All authors participated in MDS-UPDRS
design, clinimetric analysis, interpretation of results, and
manuscript writing. Dr. Goetz worked with the MDS to pro-
cure funding. Statistical analysis was conducted by Barbara
Tilley, Stephanie Shaftman, and Glenn Stebbins with input
from all other authors.
REFERENCES
1. Fahn S, Elton RL, UPDRS Program Members. Unified Parkin-
son’s disease rating scale. In: Fahn S, Marsden CD, Goldstein
M, Calne DB, editors. Recent developments in Parkinson’s dis-
ease, Vol. 2. Florham Park, NJ: Macmillan Healthcare Informa-
tion; 1987. p 153–163, 293–304.
2138 C.G. GOETZ ET AL.
Movement Disorders, Vol. 23, No. 15, 2008
2. Ramaker C, Marinus J, Stiggelbout AM, van Hilten BJ. System-
atic evaluation of rating scales for impairment and disability in
Parkinson’s disease. Mov Disord 2002;17:867–876.
3. Movement Disorder Society Task Force on Rating Scales for
Parkinson’s disease. UPDRS: status and recommendations. Mov
Disord 2003;18:738–750.
4. Goetz CG, Fahn S, Martinez-Martin P, et al. Movement disorder
society-sponsored revision of the Unified Parkinson’s disease rat-
ing scale (MDS-UPDRS): process, format, and clinimetric testing
plan. Mov Disord 2007;22:41–47.
5. Schrag A, Barone P, Brown RG, et al. Depression rating scales
in Parkinson’s disease: critique and recommendations. Mov Dis-
ord 2007;22:1077–1092.
6. Fernandez HH, Aarsland D, Fe
´
nelon G, et al. Scales to assess
psychosis in Parkinson’s disease: critique and recommendations.
Mov Disord 2008;23:484–500.
7. Grousch RL. Factor analysis, 2nd ed. Hillsdale, NJ: Lawrence
Erlbaum Associates; 1983.
8. Muthe
´
n LK, Muthe
´
n BO. Mplus users’ guide. Los Angeles:
Muthe
`
n and Muthe
`
n; 1998.
9. Brown T. Confirmatory factor analysis for applied research. New
York, NY: Guilford Press; 2006.
10. Sato K, Hatano T, Yamashiro K, et al.; Juntendo Parkinson Study
Group. Prognosis of Parkinson’s disease: time to stage III, IV, V,
and to motor fluctuations. Mov Disord 2006;21:1384–1395.
11. Levkoff S, Sanchez H. Lessons learned about minority recruit-
ment and retention from the centers on minority aging and health
promotion. Gerontologist 2003;4:18–26.
12. Stebbins GT, Goetz CG. Factor structure of the Unified Parkin-
son’s disease rating scale: motor examination section. Mov Dis-
ord 1998;13:633–636.
13. Jankovic J, Schwarz KS, Ondo W. Re-emergent tremor of
Parkinson’s disease. J Neurol Neurosurg Psychiatr 1999;67:646–650.
14. Goetz CG, Stebbins GT, Chmura TA, Fahn S, Klawans HL,
Marsden CD. Teaching tape for the motor section of the UPDRS
(with video). Mov Disord 1995;10:263–266.
2139MDS-UPDRS: CLINIMETRIC ASSESSMENT
Movement Disorders, Vol. 23, No. 15, 2008

Copyright 2008 Movement Disorder Society. All rights reserved.
This chart may not be copied, distributed or otherwise used in whole or in part without prior written consent of the Movement Disorder Society.
2140 C.G. GOETZ ET AL.

Copyright 2008 Movement Disorder Society. All rights reserved.
This chart may not be copied, distributed or otherwise used in whole or in part without prior written consent of the Movement Disorder Society.
2141MDS-UPDRS: CLINIMETRIC ASSESSMENT
Copyright 2008 Movement Disorder Society. All rights reserved.
This chart may not be copied, distributed or otherwise used in whole or in part without prior written consent of the Movement Disorder Society.
2142 C.G. GOETZ ET AL.
Copyright 2008 Movement Disorder Society. All rights reserved.
This chart may not be copied, distributed or otherwise used in whole or in part without prior written consent of the Movement Disorder Society.
2143MDS-UPDRS: CLINIMETRIC ASSESSMENT
Copyright 2008 Movement Disorder Society. All rights reserved.
This chart may not be copied, distributed or otherwise used in whole or in part without prior written consent of the Movement Disorder Society.
2144 C.G. GOETZ ET AL.
Copyright 2008 Movement Disorder Society. All rights reserved.
This chart may not be copied, distributed or otherwise used in whole or in part without prior written consent of the Movement Disorder Society.
2145MDS-UPDRS: CLINIMETRIC ASSESSMENT

Copyright 2008 Movement Disorder Society. All rights reserved.
This chart may not be copied, distributed or otherwise used in whole or in part without prior written consent of the Movement Disorder Society.
2146 C.G. GOETZ ET AL.
Copyright 2008 Movement Disorder Society. All rights reserved.
This chart may not be copied, distributed or otherwise used in whole or in part without prior written consent of the Movement Disorder Society.
2147MDS-UPDRS: CLINIMETRIC ASSESSMENT
Copyright 2008 Movement Disorder Society. All rights reserved.
This chart may not be copied, distributed or otherwise used in whole or in part without prior written consent of the Movement Disorder Society.
2148 C.G. GOETZ ET AL.
Copyright 2008 Movement Disorder Society. All rights reserved.
This chart may not be copied, distributed or otherwise used in whole or in part without prior written consent of the Movement Disorder Society.
2149MDS-UPDRS: CLINIMETRIC ASSESSMENT

Copyright 2008 Movement Disorder Society. All rights reserved.
This chart may not be copied, distributed or otherwise used in whole or in part without prior written consent of the Movement Disorder Society.
2150 C.G. GOETZ ET AL.
Copyright 2008 Movement Disorder Society. All rights reserved.
This chart may not be copied, distributed or otherwise used in whole or in part without prior written consent of the Movement Disorder Society.
2151MDS-UPDRS: CLINIMETRIC ASSESSMENT
Copyright 2008 Movement Disorder Society. All rights reserved.
This chart may not be copied, distributed or otherwise used in whole or in part without prior written consent of the Movement Disorder Society.
2152 C.G. GOETZ ET AL.

Copyright 2008 Movement Disorder Society. All rights reserved.
This chart may not be copied, distributed or otherwise used in whole or in part without prior written consent of the Movement Disorder Society.
2153MDS-UPDRS: CLINIMETRIC ASSESSMENT

Copyright 2008 Movement Disorder Society. All rights reserved.
This chart may not be copied, distributed or otherwise used in whole or in part without prior written consent of the Movement Disorder Society.
2154 C.G. GOETZ ET AL.
Copyright 2008 Movement Disorder Society. All rights reserved.
This chart may not be copied, distributed or otherwise used in whole or in part without prior written consent of the Movement Disorder Society.
2155MDS-UPDRS: CLINIMETRIC ASSESSMENT

Copyright 2008 Movement Disorder Society. All rights reserved.
This chart may not be copied, distributed or otherwise used in whole or in part without prior written consent of the Movement Disorder Society.
2156 C.G. GOETZ ET AL.

Copyright 2008 Movement Disorder Society. All rights reserved.
This chart may not be copied, distributed or otherwise used in whole or in part without prior written consent of the Movement Disorder Society.
2157MDS-UPDRS: CLINIMETRIC ASSESSMENT

Copyright 2008 Movement Disorder Society. All rights reserved.
This chart may not be copied, distributed or otherwise used in whole or in part without prior written consent of the Movement Disorder Society.
2158 C.G. GOETZ ET AL.

Copyright 2008 Movement Disorder Society. All rights reserved.
This chart may not be copied, distributed or otherwise used in whole or in part without prior written consent of the Movement Disorder Society.
2159MDS-UPDRS: CLINIMETRIC ASSESSMENT
Copyright 2008 Movement Disorder Society. All rights reserved.
This chart may not be copied, distributed or otherwise used in whole or in part without prior written consent of the Movement Disorder Society.
2160 C.G. GOETZ ET AL.
Copyright 2008 Movement Disorder Society. All rights reserved.
This chart may not be copied, distributed or otherwise used in whole or in part without prior written consent of the Movement Disorder Society.
2161MDS-UPDRS: CLINIMETRIC ASSESSMENT
Copyright 2008 Movement Disorder Society. All rights reserved.
This chart may not be copied, distributed or otherwise used in whole or in part without prior written consent of the Movement Disorder Society.
2162 C.G. GOETZ ET AL.
Copyright 2008 Movement Disorder Society. All rights reserved.
This chart may not be copied, distributed or otherwise used in whole or in part without prior written consent of the Movement Disorder Society.
2163MDS-UPDRS: CLINIMETRIC ASSESSMENT

Copyright 2008 Movement Disorder Society. All rights reserved.
This chart may not be copied, distributed or otherwise used in whole or in part without prior written consent of the Movement Disorder Society.
2164 C.G. GOETZ ET AL.

Copyright 2008 Movement Disorder Society. All rights reserved.
This chart may not be copied, distributed or otherwise used in whole or in part without prior written consent of the Movement Disorder Society.
2165MDS-UPDRS: CLINIMETRIC ASSESSMENT

Copyright 2008 Movement Disorder Society. All rights reserved.
This chart may not be copied, distributed or otherwise used in whole or in part without prior written consent of the Movement Disorder Society.
2166 C.G. GOETZ ET AL.
Copyright 2008 Movement Disorder Society. All rights reserved.
This chart may not be copied, distributed or otherwise used in whole or in part without prior written consent of the Movement Disorder Society.
2167MDS-UPDRS: CLINIMETRIC ASSESSMENT
Copyright 2008 Movement Disorder Society. All rights reserved.
This chart may not be copied, distributed or otherwise used in whole or in part without prior written consent of the Movement Disorder Society.
2168 C.G. GOETZ ET AL.

Copyright 2008 Movement Disorder Society. All rights reserved.
This chart may not be copied, distributed or otherwise used in whole or in part without prior written consent of the Movement Disorder Society.
2169MDS-UPDRS: CLINIMETRIC ASSESSMENT
Copyright 2008 Movement Disorder Society. All rights reserved.
This chart may not be copied, distributed or otherwise used in whole or in part without prior written consent of the Movement Disorder Society.
2170 C.G. GOETZ ET AL.
